Enzyme linked immunosorbent assay kit used for detecting human ox-LDL (oxidized low-density lipoprotein)
A low-density lipoprotein, enzyme-linked immunosorbent assay technology, applied in the field of medical testing, can solve the problems of poor stability of the kit, affecting large-scale applications, and difficult clinical testing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of anti-human oxidized low-density lipoprotein antibody: culture hybridoma cells (preservation number: CCTCCNO: C200304) expressing anti-OxLDL monoclonal antibody in vitro; the number of inoculated cells is 2×10 5 / ml; the composition of the culture medium was 97%DMEM / F12:RPMI1640=1:1; 3%FBS; the rolling speed was 0.5rpm; all the medium was changed once every 96 hours, and the medium was changed twice and harvested three times. And when the cells are cultured, add 10IU / 10ml LIF (leukocyte inhibitory factor), 2IU / 10ml HCG, 2IU / 10ml bovine insulin to the culture medium. The culture supernatant is filtered and clarified, purified, and the antibody is collected;
[0036] (2) Preparation of an ELISA plate coated with anti-human oxidized low-density lipoprotein antibody:
[0037] a. Antibody dilution: Dilute the anti-human oxidized low-density lipoprotein monoclonal antibody to 2.5-20 μg / ml with a 50 mM Tris-HCl buffer solution with a pH of 8.0 to obtain a co...
Embodiment 2
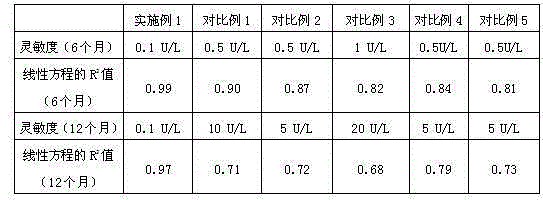
[0061] Embodiment 2 kit sensitivity measurement
[0062] Prepare PBS buffer solution of different concentrations of Ox-LDL standard substance respectively, concentration is respectively 0.1U / L, 0.5U / L, 1U / L, 5U / L, 10U / L, 20U / L, adopts the reagent prepared in embodiment 1 Cartridge is detected, with control buffer as blank control, and use contrast ratio kit to carry out the same detection at the same time, contrast ratio is the ELISA kit that patent CN201210160797 embodiment 1 prepares, and specific detection method is as follows:
[0063] a) Antigen-antibody reaction: Add 50 μl of standard solution and diluent to the microwells of the coated microtiter plate, and incubate in a water bath at 37° C. for 50 minutes. Wash the plate with washing buffer 5 times.
[0064] b) Add the HRP-labeled anti-apoB antibody solution to each well, 100 μl per well, and incubate in a 37° C. water bath for 50 minutes. Repeat the plate washing operation 5 times.
[0065] c) Chromogenic reactio...
Embodiment 3
[0071] The anti-human oxidized low-density lipoprotein antibody was prepared according to the method in Example 1 of patent CN201210160797, and the antibody content in the supernatant before purification was measured by HPLC method. At the same time, the same measurement was performed on Example 1 of the present invention, and the results showed that: Example 1 of the present invention 1 The antibody content in the harvested supernatant was 9.7 mg / L, while the antibody content in the supernatant of Patent CN201210160797 Example 1 was 3.8 mg / L.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com