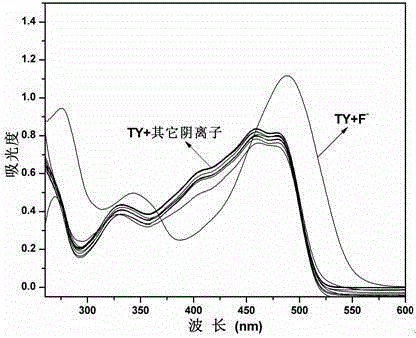
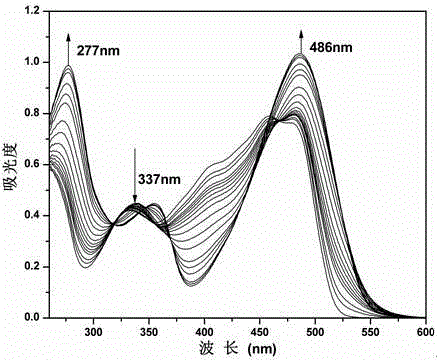
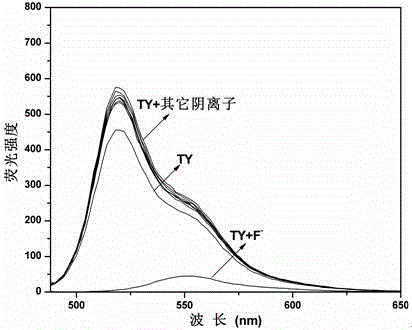
Schiff base compound capable of efficiently and individually selecting and recognizing fluorine ions as well as synthesis and application of Schiff base compound
A Schiff base and fluoride ion technology, applied in the field of anion detection and chemical synthesis, can solve the problems of Schiff base compound detection, identification of less anions, etc., and achieve the effect of good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] 1. Synthesis and characterization of compound TY
[0036] Put 0.276g (1.5mmol) of p-benzidine and 0.688g (4mmol) of 2-hydroxyl-1-naphthaldehyde in a 100mL round bottom flask, add 50mL of ethanol as a solvent, and 1mL of glacial acetic acid as a catalyst. After stirring at room temperature for 30min, Heated to reflux on an oil bath at 80°C for 4h. After the reaction was stopped and cooled to room temperature, it was filtered with suction. At the same time, it was washed three to five times with hot ethanol solution, and then recrystallized with DMF / EtOH to obtain 0.559 g of red needle-like crystals. Its synthetic formula is as follows:
[0037]
[0038] TY: Yield: 76.3%; (m.p.>300℃). 1 HNMR (DMSO-d 6 ,400MHz):δ15.87(d,2H,Ar-OH),9.72(d,2H,N=CH),8.52(d,2H,ArH),7.93-7.88(m,6H,ArH),7.78( t,6H,ArH),7.55(t,2H,ArH),7.35(t,2H,ArH),7.01(d,2H,ArH).IR(KBr,cm -1 ):v:=3431(OH),1624(C=N),1535(C=C),1487(C=C).Anal.Calcd.forC 34 h 24 N 2 o 2 : C, 82.89; H, 4.91; N, 5.69; O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com