Supermolecule bisazobenzene compound and preparation method thereof
A bisazobenzene and composite technology, applied in the field of supramolecular bisazobenzene composites and their preparation, can solve problems such as poor solubility and film-forming properties, and achieve good stability, good solubility and film-forming properties The effect of sex, wide application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
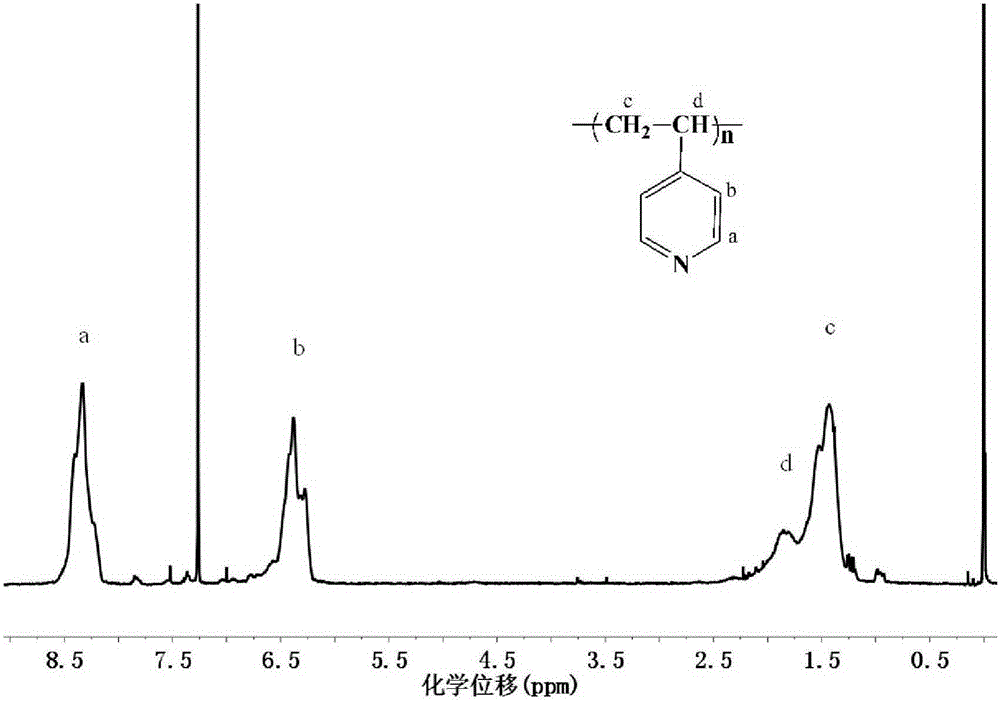
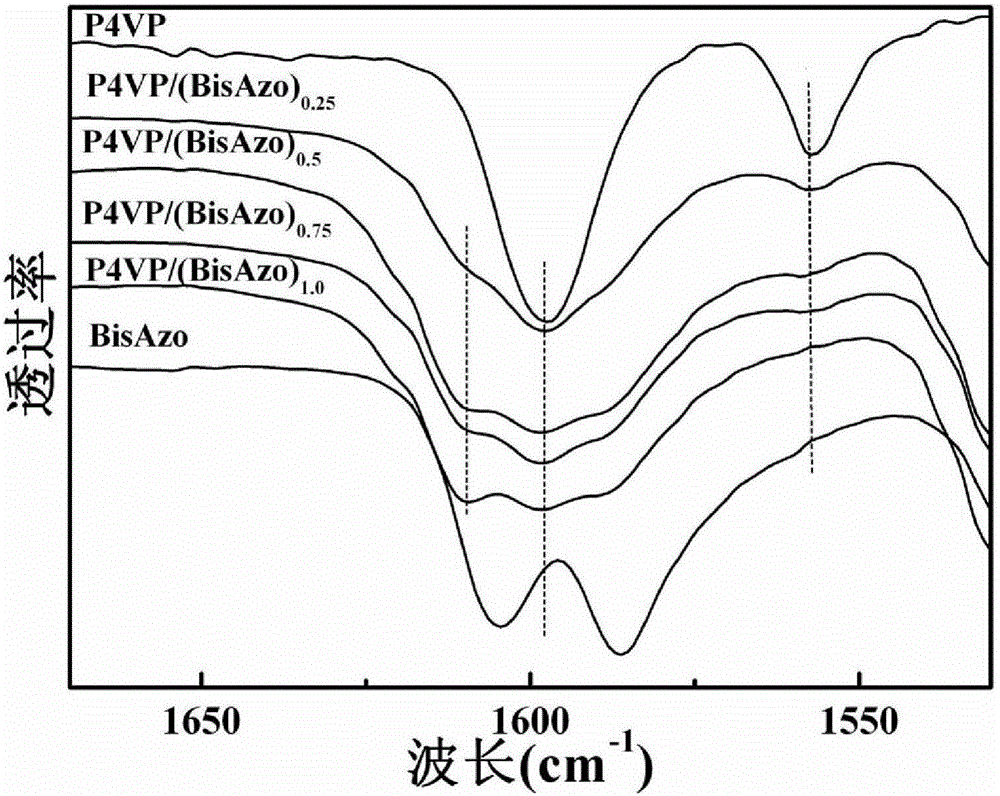
Examples
Embodiment 1
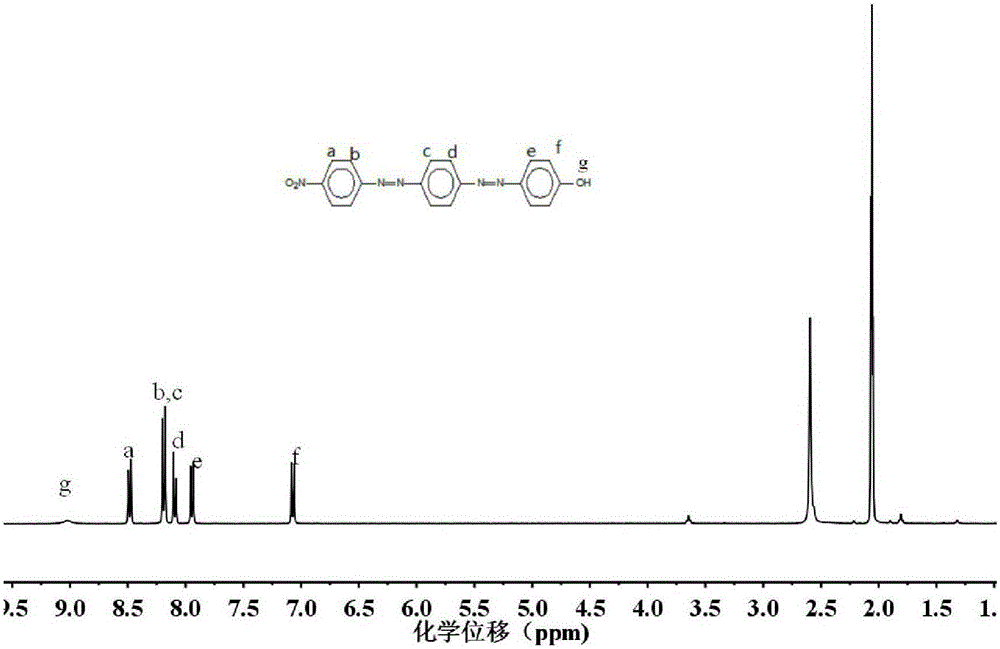
[0026] Synthesis of Small Molecules of Bisazobenzene
[0027] 1.1 Synthesis of 4-amino-4'-nitroazobenzene
[0028] In this step, the amino group of aniline is firstly protected, then 4-nitroaniline is diazotized, then the two are coupled, and finally the amino group is deprotected by hydrolysis. Concrete synthetic steps are as follows:
[0029] 1. Weigh 126g of NaHSO with a mass concentration of 20.5% 3 Solution and 17g of formaldehyde solution with a mass concentration of 37-40% were added to a 250ml single-necked bottle, and the temperature was raised to 50°C and stirred for 30 minutes; then the temperature was raised to 60°C, and 14g of aniline was added dropwise with a dropper to stop the reaction, and cooled naturally. spare;
[0030] 2. Put the beaker containing 100g of anhydrous sodium acetate and 4L of distilled water into an ice bath, add the aniline solution obtained in step 1, and stir mechanically;
[0031] 3. Weigh 25g of 4-nitroaniline into a 200ml beaker, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com