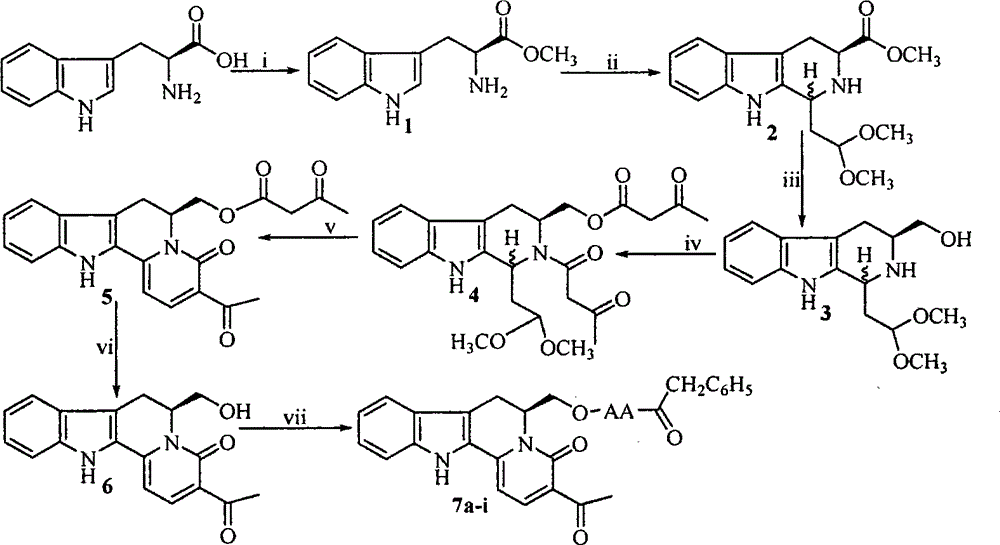
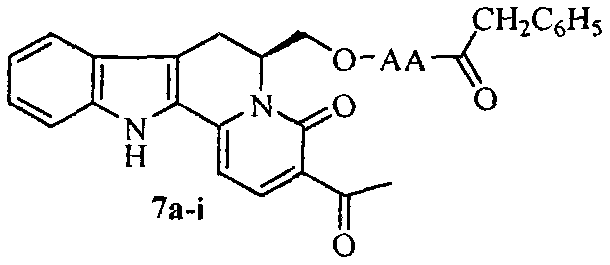
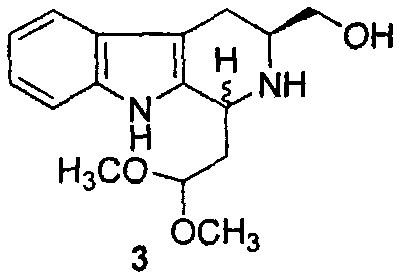
G1/G0-phase blocked pentacyclic indol-quinolizidine and compound, antineoplastic activity and application thereof
A technology of cell block and compound, applied in the field of biomedicine, can solve the problem of no blocker successful case and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 prepares L-tryptophan methyl ester hydrochloride (1)
[0021] Add 3.75ml (50mmol) thionyl chloride dropwise to 50ml of anhydrous methanol under ice bath. After the dropwise addition is completed within 30min, add 9.20g (42mmol) L-tryptophan in batches, stir at room temperature for 24h, TLC (CHCl 3 : MeOH=2:3) monitor until the disappearance of the raw material to terminate the reaction. The water pump pumps away the unreacted thionyl chloride SOCl 2 and HCl, and repeatedly triturated with ether to obtain a white solid, recrystallized from methanol-ether to obtain L-tryptophan methyl ester hydrochloride as a colorless solid. The yield is between 85-99%. ESI-MS(m / e)219[M+H] + , physical constants such as melting point and optical rotation are consistent with the reported data.
Embodiment 2
[0022] Example 2 Preparation of 1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carboline carboxylic acid methyl ester (2)
[0023] In a 250ml round bottom flask, add 20g (78.6mmol) of L-tryptophan methyl ester hydrochloride, 100ml of methanol, add 20ml of trifluoroacetic acid, activate for half an hour, then add 1,1,3,3-tetramethoxy 16ml (97.6mmol) of propane, heated to 80°C with stirring, kept the temperature for 10 hours, and the spots of the raw materials disappeared as monitored by TLC. The reaction solution was cooled to room temperature, and saturated NaHCO was added dropwise. 3 Adjust the pH of the aqueous solution to neutral, recover methanol under reduced pressure, add 100ml of dichloromethane to the residue, and extract the aqueous layer with dichloromethane (50ml×3) after separation, combine the organic layers, dry over anhydrous sodium sulfate, filter, and the filtrate It was concentrated to dryness under reduced pressure, and the residue was purified by silica gel co...
Embodiment 3
[0024] Example 3 Preparation of 1-(2,2-dimethoxyethyl)-3-hydroxymethyl-1,2,3,4-tetrahydrocarboline (3)
[0025] Add 50ml of anhydrous tetrahydrofuran into a 250ml round bottom flask, add LiAlH in batches under stirring 4 0.9g (23.6mmol), activated for half an hour. Dissolve 5g (15.7mmol) of compound (2) in 50ml of anhydrous tetrahydrofuran, then slowly drop it into the reaction flask, raise the temperature to 40°C, keep the reaction for 3 hours, and monitor the disappearance of raw material spots by TLC. Cool the reaction solution to room temperature, add 0.5ml of 15% NaOH aqueous solution, quench the reaction, filter with a Bush funnel to remove the aluminum salt, concentrate the filtrate to dryness under reduced pressure, add ethyl acetate, grind and wash with ether to obtain 2.52g (54%) Compound 3 is a colorless powder. ESI-MS(m / e)291[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com