Preparation method for benzoyldiphenyl phosphine oxide derivatives
A technology of benzoyl diphenyl and diphenyl phosphine oxide, which is applied in the field of polymers, can solve the problems of low yield and achieve the effects of increasing yield, reducing waste, and reducing reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Preparation of 2,4,6-trimethylbenzoyldiphenylphosphine oxide (TPO)
[0083] Introduce nitrogen into the reaction kettle to remove the air, add 20.2g (0.1mol) diphenylphosphine oxide, 30.3g (23mL) dichloromethane and 0.56g (0.010mol) KOH, stir, filter with suction, at room temperature Add to 34.10g (0.11mol) of 2,4,6-trimethylbenzoic anhydride organic solvent, the organic solvent is 40g of dichloromethane, after the addition, reflux at 80°C for 20h. The light yellow liquid was obtained by filtration, and then recrystallized with benzene to obtain 27.86 g of light yellow crystalline powder with a yield of 80% and a purity of 99%.
[0084] The reaction mechanism of the present embodiment is:
[0085]
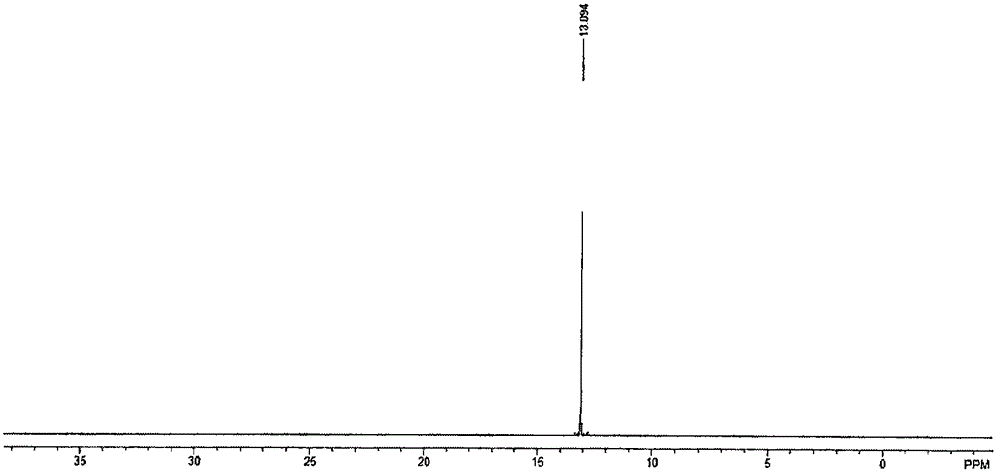
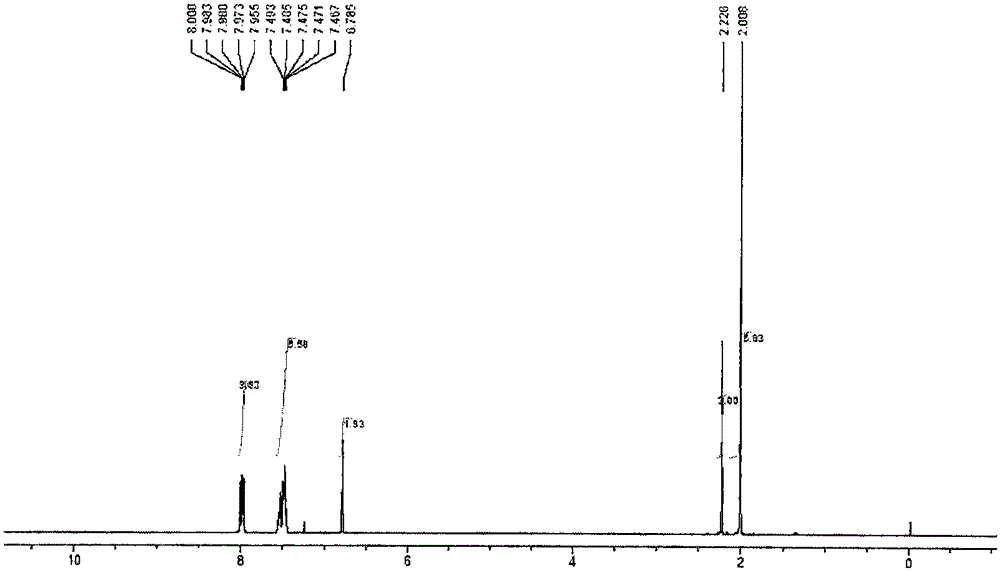
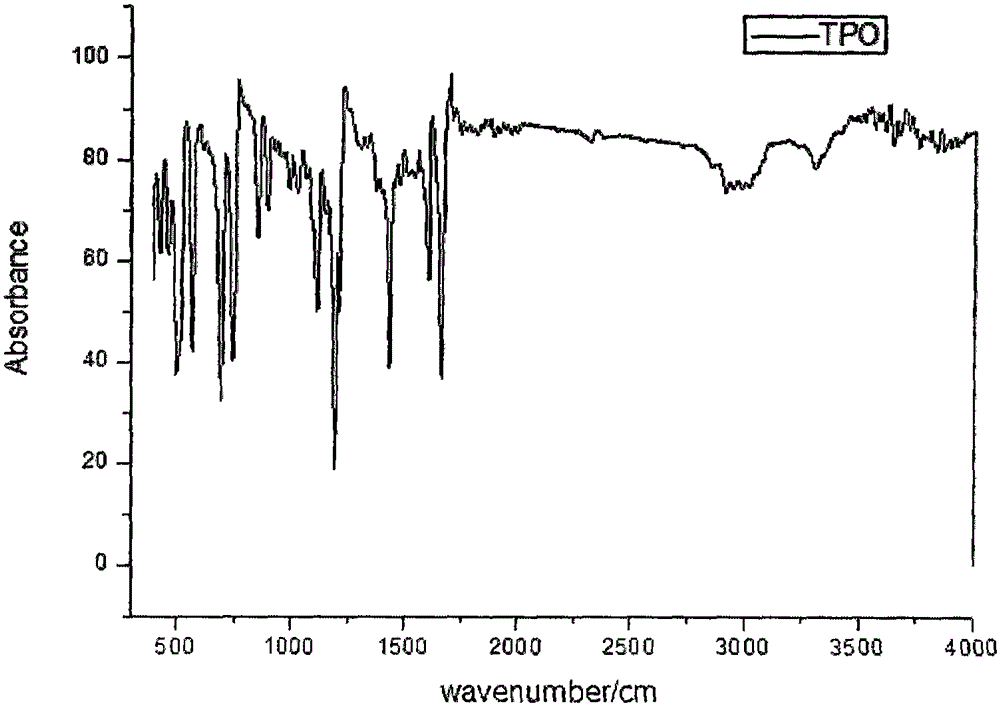
[0086] The present invention also carried out nuclear magnetic and infrared characterizations of the TPO obtained in this embodiment, and the characterization results are as follows: figure 1 , figure 1 with image 3 as shown, figure 1 For the TPO that the embodiment...
Embodiment 2
[0089] Preparation of Benzoyldiphenylphosphine Oxide
[0090] Introduce nitrogen into the reaction kettle to remove air, add 20.20g (0.1mol) diphenylphosphine oxide, 30.3g (23mL) dichloromethane and 0.62g (0.011mol) KOH, stir, and add dropwise to In the organic solvent of 24.86g (0.11mol) of benzoic anhydride, the organic solvent is 35g of dichloromethane, and after the addition is completed, the reaction is refluxed at 70°C for 20h. The light yellow liquid was obtained by filtration, and then recrystallized with petroleum ether to obtain 25.27 g of light yellow crystalline powder with a yield of 85% and a purity of more than 99%.
[0091] The product of this example was detected according to the characterization method in Example 1, and it was proved that the product obtained in this example was the target product benzoyldiphenylphosphine oxide.
Embodiment 3
[0093] Preparation of p-toluyldiphenylphosphine oxide
[0094] Introduce nitrogen into the reaction kettle to remove the air, add 20.2g (0.1mol) diphenylphosphine oxide, 30.3g (23mL) dichloromethane and 0.67g (0.012mol) KOH, stir, filter with suction, and Add to 27.94g (0.11mol) of p-toluic anhydride organic solvent, the organic solvent is 37g of toluene, after the addition, reflux at 75°C for 20h, filter to obtain a light yellow liquid, and then use petroleum ether for recrystallization , to obtain 26.72g of light yellow crystalline powder with a yield of 85% and a purity of 99%.
[0095] The product of this example was detected according to the characterization method in Example 1, and it was proved that the product obtained in this example was the target product p-toluyldiphenylphosphine oxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com