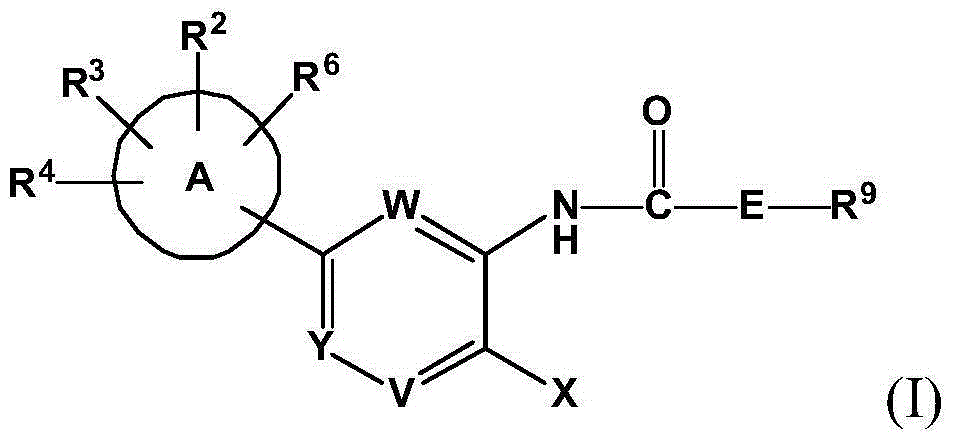
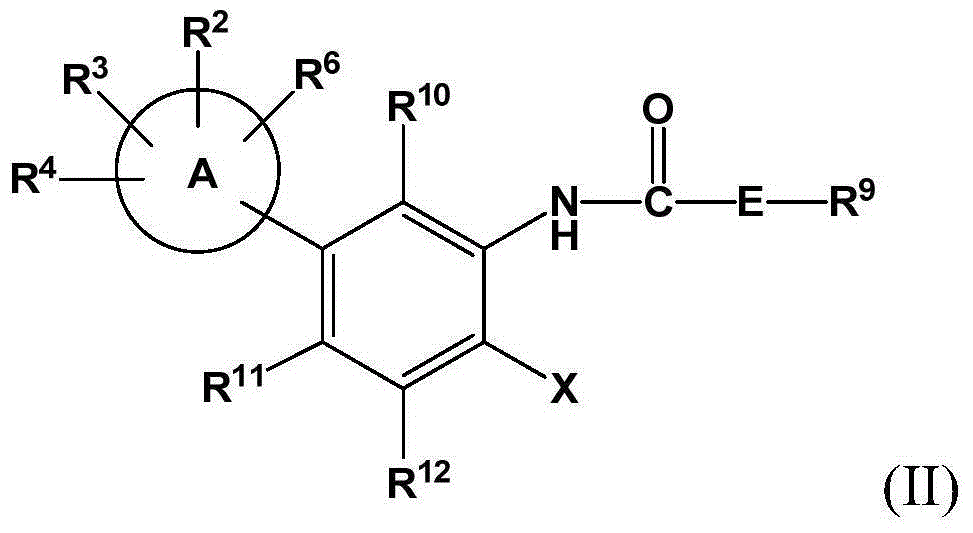
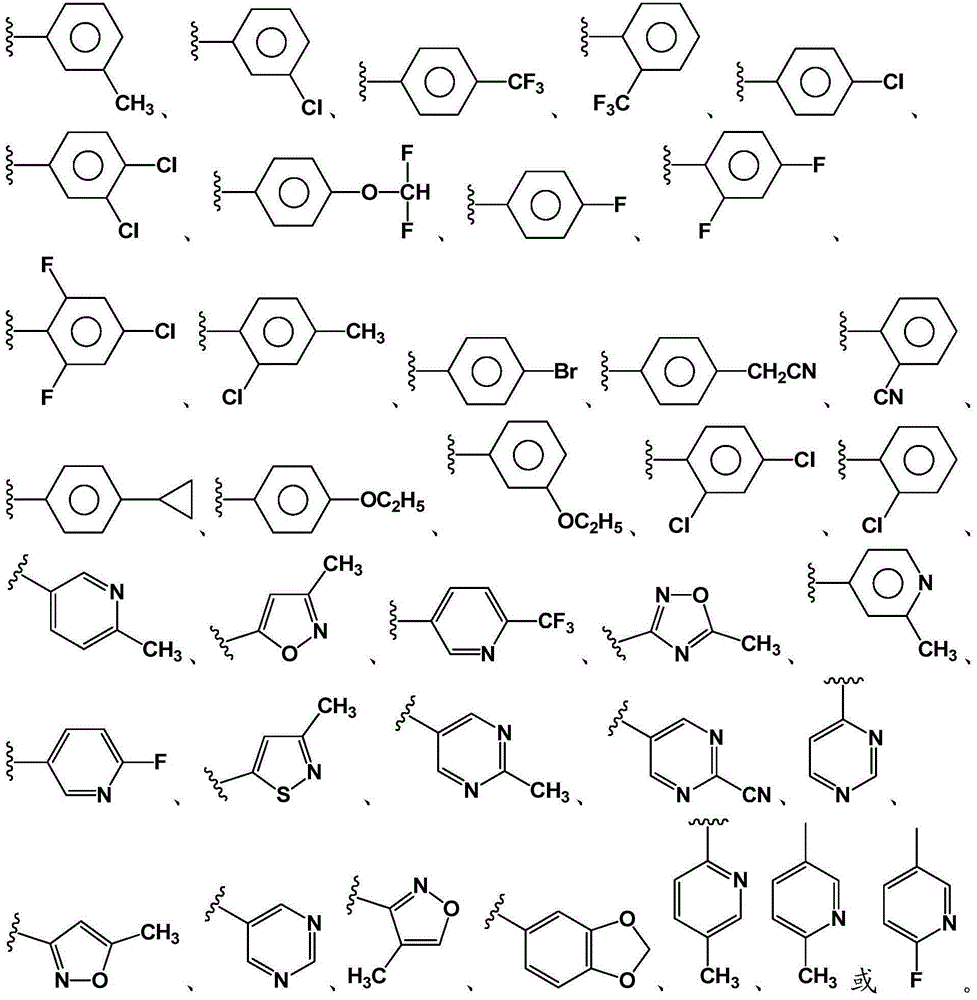
Inhibitors of indoleamine 2,3-dioxygenase (IDO)
A C3-C8, C2-C10 technology, applied in the field of enzyme active compounds, can solve the problem of increased tolerance of the tumor microenvironment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0223] The preparation of prodrugs is well known in the art and described, for example, in King, F.D., ed., Medicinal Chemistry: Principles and Practice, The Royal Society of Chemistry, Cambridge, UK (2 nd edition, reproduced, 2006); Testa, B.etal., Hydrolysis in Drug and Prodrug Metabolism. Chemistry, Biochemistry and Enzymology, VCHA and Wiley-VCH, Zurich, Switzerland (2003); Wermuth, C.G., ed., The Practice of Medicinal Chemistry, 3 rd edition, Academic Press, San Diego, CA (2008).
[0224] The present invention is intended to include all isotopes of atoms present in the compounds of the present invention. Isotopes include atoms with the same atomic number but different mass numbers. By way of general example and not limitation, isotopes of hydrogen include deuterium and tritium. Isotopes of carbon include 13 C and 14 c. Isotopically-labeled compounds of the invention can be prepared generally by conventional techniques known to those skilled in the art or by methods a...
Embodiment 1
[0386] Racemic Example 1. Racemic (1R,2S)-2-(4-(diisobutylamino)-3-(3-(p-tolyl)ureido)phenyl)cyclopropanecarboxylic acid
[0387] To a solution of 1D (140 mg, 0.421 mmol) in THF (4 mL) was added 1-isocyanato-4-methylbenzene (0.079 mL, 0.632 mmol). The resulting solution was stirred at room temperature for 3 h. LC-MS indicated completion. The reaction mixture was concentrated and used in the next step without purification. The crude ester (180 mg, 0.387 mmol) was dissolved in THF (4 mL), and NaOH (1N aq) (1.160 mL, 1.160 mmol) was added. MeOH (1 mL) was then added to dissolve the precipitate and it became a clear yellow solution. After 60 h, LC-MS indicated that the reaction was complete. Most of the MeOH and THF were removed in vacuo and the crude was diluted with 2 mL of water and the pH was adjusted to ~2 using 1N aqueous HCl. The aqueous phase was then extracted with EtOAc (3×10 mL) and the combined organic phases were washed with brine, washed with Na 2 SO 4 Dried a...
Embodiment 2-16
[0425]
[0426] Following the procedure of Method C of Enantiomer 1 of Example 1, using the corresponding isocyanate, Examples 2-16 were prepared.
[0427]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com