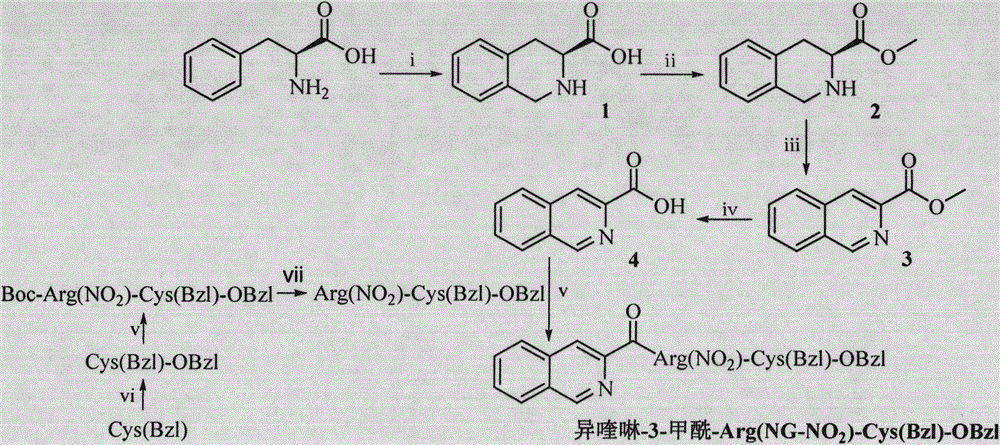
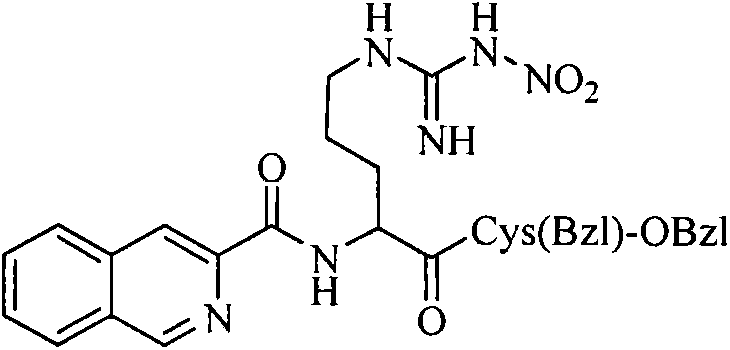
Isoquinoline-3-formyl-RC-OBzl, and preparation, nanometer structure, activity and application thereof
A nanostructured, isoquinoline technology, used in the preparation of antithrombotic drugs, isoquinoline-3-formyl-Arg-Cys-OBzl, antithrombotic activity, its nano-resultant field, can solve cytotoxicity And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 prepares tetrahydroisoquinoline-3-carboxylic acid
[0021] Weigh 1g of L-phenylalanine, add 2.4mL of formaldehyde, and then add 8mL of 35% hydrochloric acid, the solution is first clarified and then a white substance precipitates out, and the reaction is carried out in an oil bath at 80°C for 8 hours. After the reaction, the solvent was removed and placed in an eggplant bottle. Diethyl ether was added and ultrasonically stirred for 1 hour, repeatedly ground and washed, and filtered to obtain 0.91 g (85%) of tetrahydroisoquinoline-3-carboxylic acid as a colorless solid. The melting point is 246-247°C. ESI-MS(m / e): 176[M-H] - .
Embodiment 2
[0022] Embodiment 2 prepares methyl tetrahydroisoquinoline-3-carboxylate
[0023] Add 6ml of methanol and 0.4ml of thionyl chloride to a 50ml eggplant-shaped bottle, stir, activate for 30min, and ice-bath. Add 239 mg of tetrahydroisoquinoline-3-carboxylic acid, and react under ice-cooling for 12 hours. The solvent was drained, methanol was added, and the solvent was drained 3 times; ether was added 3 times, and the solvent was drained to obtain 213 mg (82.5%) of methyl tetrahydroisoquinoline-3-carboxylate as a colorless powder. ESI-MS(m / e): 192[M+H] + .
Embodiment 3
[0024] Embodiment 3 prepares methyl isoquinoline-3-carboxylate
[0025] Dissolve 213 mg of methyl tetrahydroisoquinoline-3-carboxylate in anhydrous DMF, add potassium permanganate solution dissolved in distilled water, add slowly under ice bath, and react at room temperature for 24 hours. After the reaction, the DMF was blown dry, the black powder was dissolved with methanol and then filtered, and the filtrate was spin-dried. Separation and purification by column chromatography. 77 mg (37%) of methyl isoquinoline-3-carboxylate were obtained as a yellowish solid. Rf=0.25 (dichloromethane:methanol, 20:1); ESI-MS (m / e): 188[M+H] + ; 1 HNMR (DMSO-d 6 , 500MHz) δ / ppm=9.421(s, 1H), 8.666(s, 1H), 8.258(d, J=4Hz, 1H), 8.22(d, J=4Hz, 1H), 7.897(m, 2H), 3.944 (s, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com