Catalytic composition and process for the dimerisation of ethylene to 1-butene
一种组合物、化合物的技术,应用在碳氢化合物、碳氢化合物、化学仪器和方法等方向,能够解决催化剂失活可操作性难度等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
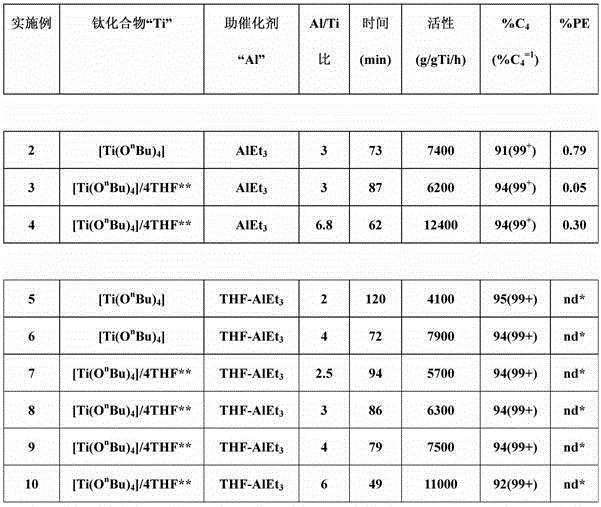
[0042] Example 1: Adduct "THF.AlEt 3 "Synthesis
[0043] Dissolved 0.832 g (7.3 mmol) of AlEt in an inert atmosphere 3 Introduce 25 mL of n-heptane into the Schlenk flask. Next, 0.54 g (7.5 mmol) of THF were added in a controlled manner. The solution was stirred at ambient temperature for about 1 hour. The n-heptane was then removed under dynamic vacuum at ambient temperature. Adduct "THF.AlEt 3 ” was isolated as a colorless liquid in near quantitative yield (95%). 1 HNMR analysis confirmed THF / AlEt 3 The molar ratio is 1.03 / 1.
[0044] 1 HNMR: (300MHz, CD 2 Cl 2 ); δ (ppm): 4.12 (m, 4H); 2.11 (m, 4H); 1.04 (t, 9H); -0.18 (q, 6H).
Embodiment 2-10
[0046] The ethylene dimerization experiments given in Table 1 below were carried out in a stainless steel autoclave with an effective volume of 500 ml, equipped with a jacket for temperature regulation by oil circulation. Agitation is provided using a Rushton impeller with mechanical drive. 40 ml of n-heptane and 5 ml of a 0.085 mol / l solution of a titanium compound in n-heptane were introduced into the reactor under an ethylene atmosphere at ambient temperature. Once the temperature of the reactor reached 53°C, the required amount of aluminum-based cocatalyst (diluted in n-heptane) was introduced under ethylene pressure. For Examples 2-4 (comparative examples), the aluminum-based cocatalyst is AlEt 3 . For Examples 5-10 (according to the invention), the aluminum-based cocatalyst is the adduct THF-AlEt synthesized in Example 1 3 . The ethylene pressure was maintained at 23 MPa, and the temperature was maintained at 53°C. After a reaction time "t" (see Table 1), the introd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com