Growth hormone secretagogue receptor based protein, nucleic acids and methods and uses thereof
A technology for secretagogues and growth hormones, applied in the field of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
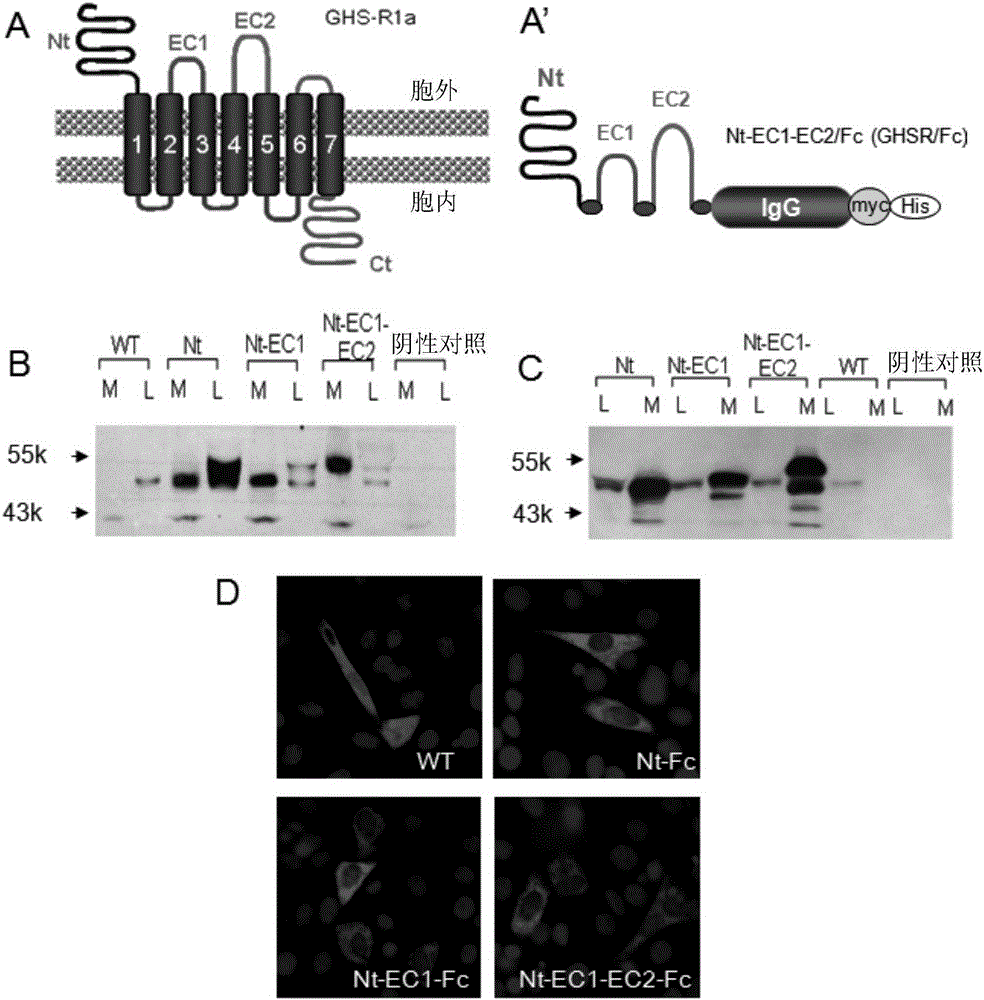
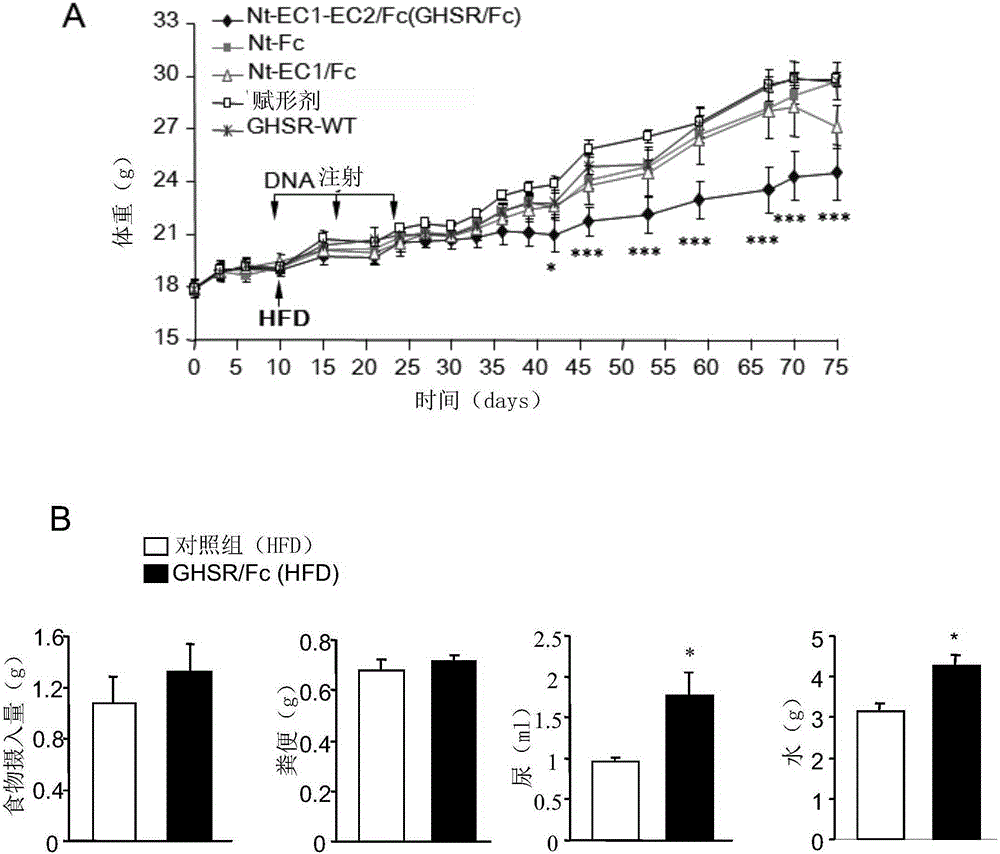
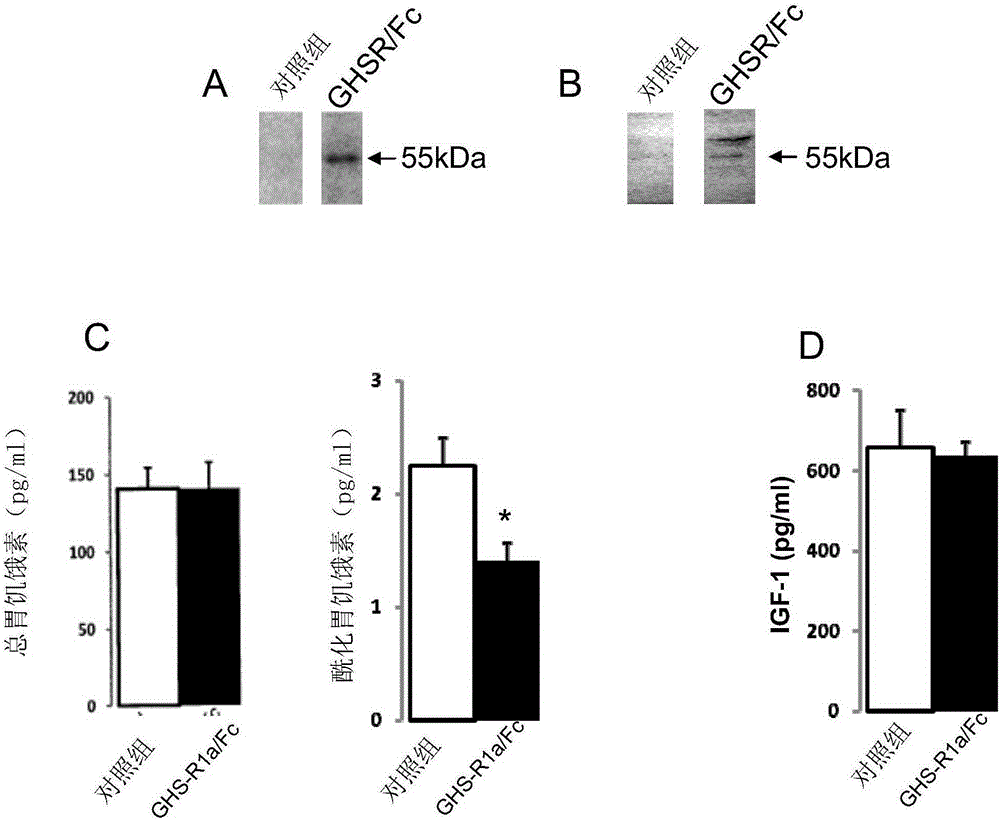
[0101] Attenuation of circulating ghrelin may reduce calorie intake and promote fat energy utilization. In this way, a mammalian expression plasmid encoding the ligand-binding region of GHS-R1a, particularly the N-terminal (Nt) and / or extracellular loops 1 and 2 (EC1, EC2) was constructed and linked to the murine IgG constant region (Fc) Fusion, composed of GHSR / Fc( figure 1 A). These fusion proteins were successfully expressed in vivo by intramuscular injection followed by electrotransfer in mouse gastrocnemius muscle tissue. The fusion molecule omits the sequence corresponding to the transmembrane domain of the receptor, so that the product of GHSR / Fc can be secreted extracellularly.
[0102] result
[0103] Effectiveness of the GHSR molecule
[0104] First, through the transient expression of the plasmid vector ( figure 1 A) Confirm the expression and secretion of the fusion protein in rat skeletal muscle cell L6 under in vitro conditions. 48 hours after transfection,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com