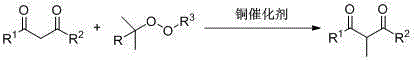Method for preparing 2-methyl-1,3-dicarbonyl derivative
A technology of dicarbonyl and derivatives, which is applied in the field of preparation of organic compounds, can solve the problems of unfavorable environment of chemical reagents, harsh reaction conditions, inconvenient operation, etc., and achieve the effect of being conducive to safe production, easy availability of raw materials, and avoiding waste of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Synthesis of 2-methyl-1,3-diphenyl-1,3-propanedione
[0051] Using 1,3-diphenyl-1,3-propanedione and tert-butyl peroxybenzoate as raw materials, the reaction steps are as follows:
[0052] Add 1,3-diphenyl-1,3-propanedione (0.22g, 1mmol), tert-butyl peroxybenzoate (0.58g, 3mmol), CuCl (0.01g, 0.1mmol) and 2mL acetic acid, react at 120°C;
[0053] TLC tracking reaction until complete completion;
[0054] The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 40:1) to obtain the target product (90% yield). The analytical data of the product are as follows: 1 HNMR (400MHz, CDCl 3 ): δ 7.98 (dt, J =8.6,1.7Hz,4H),7.70–7.52(m,2H),7.53–7.41(m,4H),5.30(q, J =7.0Hz,1H),1.63(d, J =7.0Hz, 3H).
Embodiment 2
[0055] Example 2: Synthesis of 1-(4-methylphenyl)-2-methyl-3-phenyl-1,3-propanedione
[0056] Using 1-(4-methylphenyl)-3-phenyl-1,3-propanedione and tert-butyl peroxybenzoate as raw materials, the reaction steps are as follows:
[0057] Add 1-(4-methylphenyl)-3-phenyl-1,3-propanedione (0.24 g, 1 mmol), tert-butyl peroxybenzoate (0.58 g, 3 mmol), CuCl (0.01g, 0.1mmol) and 2mL acetic acid, react at 110°C;
[0058] TLC tracking reaction until complete completion;
[0059] The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 40:1) to obtain the target product (yield 85%). The analytical data of the product are as follows: 1 HNMR (400MHz, CDCl 3 ): δ 7.97 (dd, J =5.2,3.4Hz,2H),7.89(d, J =8.2Hz,2H),7.61–7.53(m,1H),7.46(t, J =7.7Hz,2H),7.27(d, J =8.2Hz,2H),5.27(q, J =7.0Hz,1H),2.42(s,3H),1.61(d, J =7.0Hz, 3H).
Embodiment 3
[0060] Example 3: Synthesis of 1-(4-methoxyphenyl)-2-methyl-3-phenyl-1,3-propanedione
[0061] Using 1-(4-methoxyphenyl)-3-phenyl-1,3-propanedione and tert-butyl hydroperoxide as raw materials, the reaction steps are as follows:
[0062] Add 1-(4-methoxyphenyl)-3-phenyl-1,3-propanedione (0.25 g, 1 mmol), tert-butyl hydroperoxide (0.27 g, 3 mmol), CuCl (0.01g, 0.1mmol) and 2mL acetic acid, react at 100°C;
[0063] TLC tracking reaction until complete completion;
[0064] The crude product obtained after the reaction was separated by column chromatography (petroleum ether:ethyl acetate=40:1) to obtain the target product (yield 88%). The analytical data of the product are as follows: 1 HNMR (400MHz, CDCl 3 ): δ 8.08–7.76(m,4H),7.56(dd, J =10.5,4.2Hz,1H),7.45(t, J =7.7Hz,2H),7.10–6.73(m,2H),5.23(q, J =7.0Hz,1H),3.86(s,3H),1.60(d, J =7.0Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com