Method for preparing analgesic polypeptide from silkworms
A silkworm and active polypeptide technology, applied in the field of genetic engineering, can solve the problems of high cost of conotoxins, inability to solve C-terminal amidation, complex modification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Conotoxins ω- Synthesis of MVIIA Gene and Construction of Recombinant BmNPV
[0021] Bombyx mori nuclear polyhedrosis virus (BmNPV) poh , gp64 , VP39 Based on the analysis of codon usage frequency of highly expressed genes (GenBank: L33180), the codons of the conotoxin ω-MVIIA gene (GenBank: FJ959111) were optimized (see figure 1 ), the sequence of the codon-optimized ω-MVIIA gene is SEQ ID NO.1, and the sequence of the ω-MVIIA precursor protein encoded by it is SEQ ID NO.2.
[0022] The ω-MVIIA gene (synthesized by Shanghai Jierui Biotechnology Co., Ltd.) was synthesized by chemical methods, and the Bam HI / Hind After III double digestion, the donor plasmid pFastBacDual (purchased from Invitrogen) that had been cut with the same restriction enzyme was connected to form the recombinant plasmid pFBD-MVIIA. Using the primer pair P1 / P2 (SEQIDNO.3; SEQIDNO.4) to amplify the green fluorescent protein (EGFP) gene, the Kpn I / xho After double enzy...
Embodiment 2
[0024] Example 2: Expression and analgesic activity analysis of recombinant ω-MVIIA
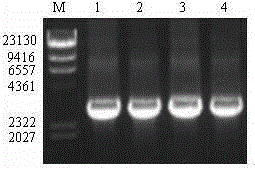
[0025] Extracted vBm by liposome method MⅦA / EGFP The silkworm cells cultured in vitro were transfected with DNA, and after 5 days of culture, green fluorescence could be observed under a fluorescent microscope. Collect the cell culture, take the supernatant after centrifugation, transfer it to the silkworm cell cultured in vitro again, inoculate and amplify the virus repeatedly, and a strong fluorescent signal can be observed under a fluorescent microscope. Determination of recombinant virus vBm with green fluorescence by endpoint dilution MVIIA / EGFP The titer of the BV particles was 1.6 x 10 7 pfu / mL.
[0026] Appropriately dilute the high-titer recombinant virus solution, inject the 5th instar silkworm with 200pfu BV particles, and after 5 days of infection, the recombinant virus vBm MⅦA / EGFP The body surface of the infected silkworm showed strong green fluorescence, while the ω-MVIIA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com