Transgenic vector for target mutation of primordial germ cells, method for preparing transgenic vector and application thereof
A technology of primordial germ cells and transgenic vectors, applied in the direction of using vectors to introduce foreign genetic material, cells modified by introducing foreign genetic material, recombinant DNA technology, etc. The effect of saving cost and effort, reducing phenotypic effects, and increasing embryo survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A transgenic vector pTol2 (UAS:Cas9) for targeted mutation of primordial germ cells, prepared by the following steps
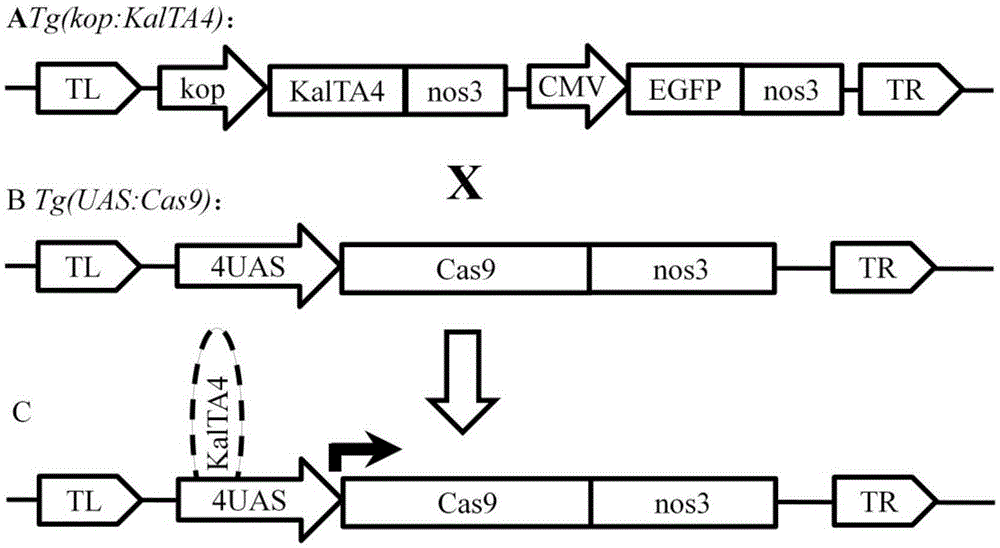
[0041] 1. Design a transgenic line that specifically expresses Cas9 in zebrafish primordial germ cells based on the Gal4 / UAS system, see the strategy diagram figure 1 As shown, then design the primers needed to amplify each fragment in the transgenic vector. The primer sequences are shown in the table below:
[0042]
[0043] 2. Construction of the transgenic vector pTol2 (UAS:Cas9).
[0044] The plasmids pBK-KalTA4 (Distel, M., Wullimann, M.F. and Koster, R.W. (2009). Optimized Gal4 genetics for permanent gene expression mapping inzebrafish. ProcNatlAcadSciUSA106, 13365-70.) and pGH-T7-zCas9 (Liu, D., Wang, Z., Xiao, A., Zhang, Y., Li, W., Zu, Y., Yao, S., Lin, S. and Zhang, B. (2014). Efficient gene targeting in zebra fish mediated by aze bra fish-codon-optimized cas 9 and evaluation of off-targeting effect. J Genet Genomics 41, 43-6.) As a temp...
Embodiment 2
[0052] A method for preparing a transgenic zebrafish Tg (UAS:Cas9) strain, comprising:
[0053] Tol2 transposase mRNA was synthesized using Ambion's MACHINE in vitro transcription kit, and then the transgenic vector pTol2 (UAS:Cas9) was mixed with the Tol2 transposase mRNA synthesized by in vitro transcription at a ratio of 1:5 (total concentration 100ng / l) , and introduced it into single-cell stage zebrafish embryos by microinjection to obtain P0 generation transgenic fry.
[0054] Collect P0 generation embryos, use primers F: GATCCCATCGCGTCTCAG, R: CCCAGCCCACGCTATTTG, and perform PCR to detect the transmission of the transgene. The amplification conditions are: 95°C for 4 minutes; 95°C for 30 seconds; 57°C for 30 seconds; 72°C Extend for 90 seconds, 30 cycles; extend at 72°C for 10 minutes; store at 4°C.
[0055] The F1 generation was bred by crossing the P0 generation of positive individuals with the wild-type zebrafish. The F1 population was cultured to sexual maturity, ...
Embodiment 3
[0057] Embryos of Tg(kop:KalTA4) females and Tg(UAS:Cas9) males specifically express Cas9 in primordial germ cells:
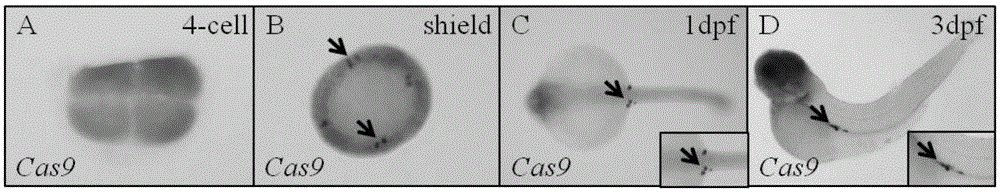
[0058] 1. In situ hybridization detection Cas9 was specifically expressed in primordial germ cells in embryos hybridized between Tg(kop:KalTA4) female fish and Tg(UAS:Cas9) male fish.
[0059] (1) Cas9 probe preparation.
[0060] A. Template preparation and purification.
[0061] Using plasmid pTol2 (UAS:Cas9) as template, zCas9-probe-F: ACTGAAAGGAAGCCCCGAG and T3-nanos3-R: GATCCATTAACCCTCACTAAAGGGAATCTCCCGGAGCATCAAT as primers, PCR amplified template DNA, referring to Axygen’s PCRcleanup kit to recover the PCR product, which was used as Synthetic probes for probes.
[0062] B. Probe synthesis and recovery.
[0063] RNA probes were transcribed and synthesized according to Promega's T3 RNA polymerase instructions, and the probes were recovered according to the instructions of sigmaspinpost-reactionchean-upcolumns.
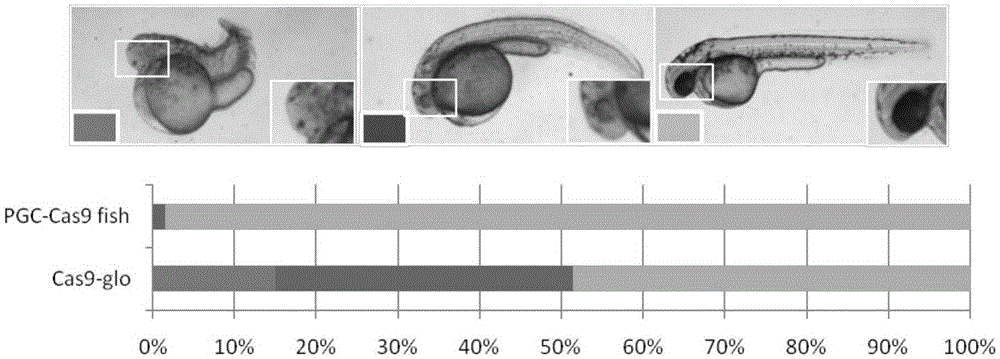
[0064] (2) In situ hybridization was used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com