Preparation method for high-nickel material surface coating layer
A surface coating and high-nickel technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of high equipment requirements, poor repeatability, cumbersome coating process, etc., to reduce side reactions, reduce the amount of residual alkali on the surface, Effect of improving cycle stability and storage performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Testing LiNi by Potentiometric Titrator 0.85 co 0.1 Al 0.05 o 2 The total base number of the material, the H needed to calculate the residual lithium on the precipitation surface 3 BO 3 The amount of precipitant is prepared to have a solution concentration of 0.1mol / L. 1000gLiNi 0.85 co 0.1 Al 0.05 o 2 Dissolve in 400g deionized water and stir to form a slurry, place it in the reaction kettle, and add H 3 BO 3 The solution was stirred and reacted in the reactor, and the dropwise addition was stopped when the pH of the solution was 11.6, and the stirring was continued for 30 minutes. The resulting suspension was then evaporated to remove water at 120°C. The obtained dry powder was heated to 700 °C in an oxygen-containing atmosphere, kept at this temperature for 10 hours, and then naturally cooled to room temperature to obtain Li 3 BO 3 Surface coated modified materials.
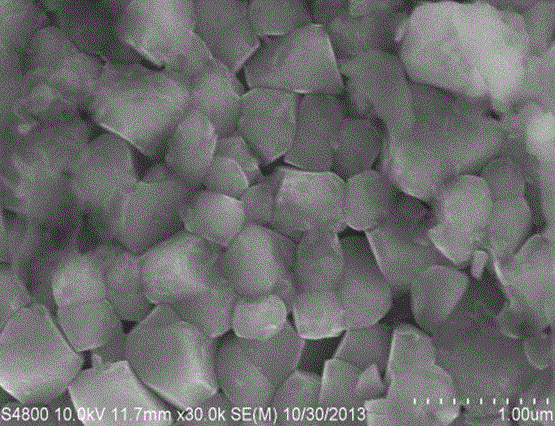
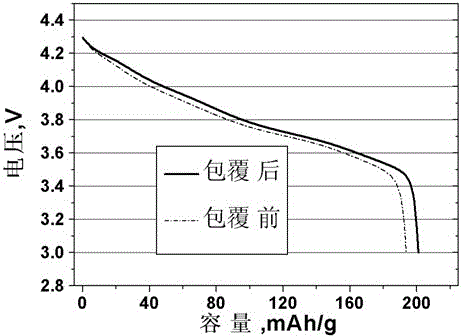
[0023] The SEM images of the high-nickel material before and after surface coating are...
Embodiment 2
[0025] Adopt the method identical with embodiment 1 to calculate LiNi 0.8 co 0.1 mn 0.1 o 2 NH required for residual lithium on the surface 4 F dosage, prepare its solution concentration to be 0.2mol / L. 1000gLiNi 0.8 co 0.1 mn 0.1 o 2 Dissolve in 400g of deionized water and stir to form a slurry, which is placed in the reaction kettle, and NH 4 The F solution was added dropwise to the reactor, and the dropwise addition was stopped when the pH of the solution was 11.5. The obtained suspension was not washed, and the drying method was stirring and drying in a water bath. The obtained dry powder was heated to 650° C. in an oxygen-containing atmosphere, kept for 8 hours, and then naturally cooled to room temperature to obtain a LiF surface-coated modified material.
Embodiment 3
[0027] Adopt the method identical with embodiment 1 to calculate LiNi 0.8 co 0.2 o 2 Requirements for residual lithium on the surface (NH 4 ) 2 HPO 4 Dosage, the preparation of its solution concentration is 0.15mol / L. 1000gLiNi 0.8 co 0.2 o 2 Dissolve in 400g deionized water and stir to form a slurry, which is placed in a reactor, and simultaneously (NH 4 ) 2 HPO 4 The solution was added dropwise to the reaction kettle, and the dropwise addition was stopped when the pH of the solution was 11.2, mechanically stirred at room temperature for 30 minutes, then filtered and washed, and the sample was dried in a vacuum oven at 120°C for 10 hours. The obtained material was heated to 750 °C in an oxygen-containing atmosphere, kept at this temperature for 8 hours, and then naturally cooled to room temperature to obtain Li 3 PO 4 Surface coated modified materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com