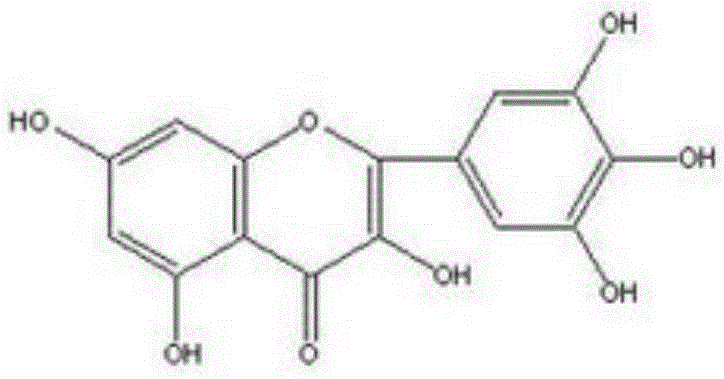
Preparation method of myricetin
A technology of myricetin and dihydromyricetin, applied in the direction of organic chemistry, etc., to achieve the effects of simple operation, low cost and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The semi-synthetic method of myricetin of the present invention comprises following synthetic route:
[0017]
Embodiment 1
[0019] In a four-necked reaction flask, feed 20 g of dihydromyricetin, 200 ml of ethanol with a mass concentration of 85%, stir evenly, add dropwise 13 ml of sodium hypochlorite solution with a mass concentration of 10%, 1 g of anhydrous ferric chloride, and heat up to reflux for 2 h Finally, add 2ml of sodium hypochlorite solution with a mass concentration of 10%, and continue to reflux. After 2h, TLC detects that the reaction of the raw materials is complete, and the reaction is stopped. It is cooled to room temperature, left overnight, filtered, and dried at 60°C to obtain 17g of yellow needle-shaped solids. Content 98% (HPLC), yield 85.5%.
Embodiment 2
[0021] In a four-necked reaction flask, feed 20 g of dihydromyricetin, 260 ml of ethanol with a mass concentration of 90%, stir well, add dropwise 12 ml of sodium hypochlorite solution with a mass concentration of 10%, 1 g of anhydrous ferric chloride, and heat up to reflux for 1 h Finally, add 2.5ml of sodium hypochlorite solution with a mass concentration of 10%, and continue to reflux. After 2.5 hours, TLC detects that the reaction of the raw materials is complete, stop the reaction, cool down to room temperature, stand overnight, filter, and dry at 60°C to obtain a yellow needle-shaped solid 17.4g. Content 98% (HPLC), yield 87.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com