A combination drug for treating pain, its preparation and preparation method
A drug, pharmacy technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
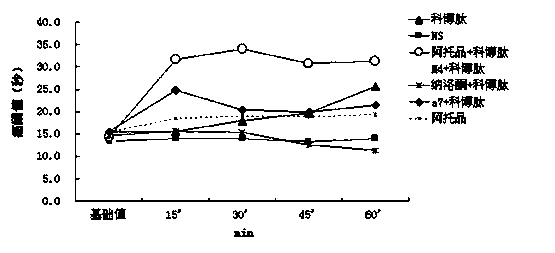
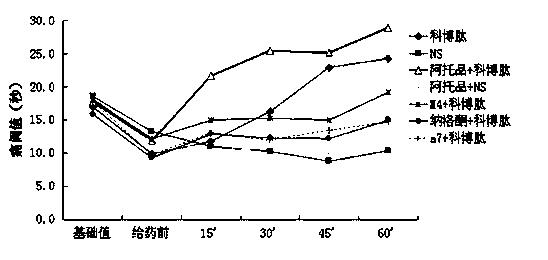
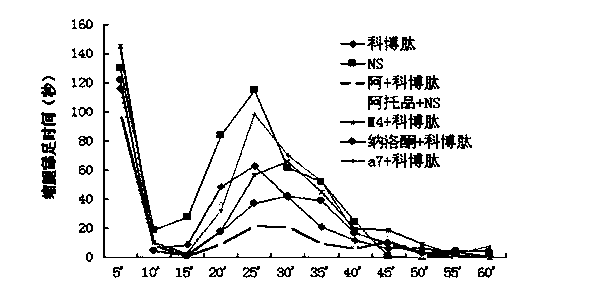
[0028] Experiment 1 Analgesic study of combined drugs
[0029] 1.1 Normal mouse hot plate experiment:
[0030] Put healthy mice (20±2g) with basically the same physical signs on a metal plate heated to 56±1°C, thermally stimulate the feet of the mice to produce pain and licking reactions. The latency of hind paw licking reaction in mice was used as the pain response index, which was the pain threshold. Mice with hind paw licking latency 30 s and jumping were excluded. Before administration, the pain threshold of each mouse was measured three times and the average value was taken as its basic pain threshold.
[0031] Administration method:
[0032] Experimental group 1-4: mice were given intraperitoneal injection of 0.5 μg atropine, tail vein injection of 0.15 μg naloxone, tail vein injection of 0.01 μg tropicamide (M4 muscarinic receptor antagonist), tail vein injection of 0.1 μg Methyllycaconitine (MLA, α7 nicotinic receptor antagonist) was administered in a volume of 0.1...
Embodiment 1
[0079] Example 1 Preparation of Injectable Microemulsion Containing Cobotide and Atropine (Injection Microemulsion A1)
[0080] Dissolve 40 mg of active ingredient cobotide and 20 mg of atropine in 300 ml of water for injection, add 6 g of poloxamer, and stir evenly to obtain an aqueous phase solution; heat 600 ml of soybean oil for injection at 100-110°C, add 5 g of poloxamer Magnesium monostearate, stir until the oil is clear and transparent, keep it warm at 105°C for 30 minutes, place it at room temperature to cool to obtain an oil phase solution; then pour the prepared water phase solution into the prepared oil phase solution, and make up the oil for injection to 1000ml, stir (2000 rpm) for 30 minutes, after homogenization, water-in-oil oil emulsion is obtained, which is divided into ampoules, sterilized, and injection microemulsion is obtained, each 2.5ml, containing the effective amount of cobotide 100μg, atropine 50μg.
Embodiment 2
[0081] Example 2 Preparation of Injectable Microemulsion Containing Cobotide and Atropine (Injection Microemulsion A2)
[0082]Dissolve 20 mg of kobotide and 8 mg of atropine in 280 ml of water for injection, add 2 g of Span-60, and stir well to obtain an aqueous phase solution; take 600 ml of soybean oil for injection and heat it at 100-110 ° C, add 2 g of Span-60 Magnesium monostearate, stir until the oil is clear and transparent, keep it warm at 105°C for 30 minutes, place it at room temperature to cool to obtain an oil phase solution; then pour the prepared water phase solution into the prepared oil phase solution, and make up the oil for injection to 1000ml, stir (2000 rpm) for 30 minutes, after homogenization, water-in-oil oil emulsion is obtained, which is divided into ampoules, sterilized, and injection microemulsion is obtained, each 2.5ml, containing the effective amount of cobotide 50μg, atropine 20μg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com