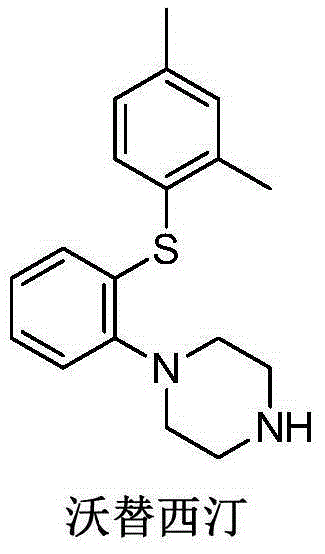
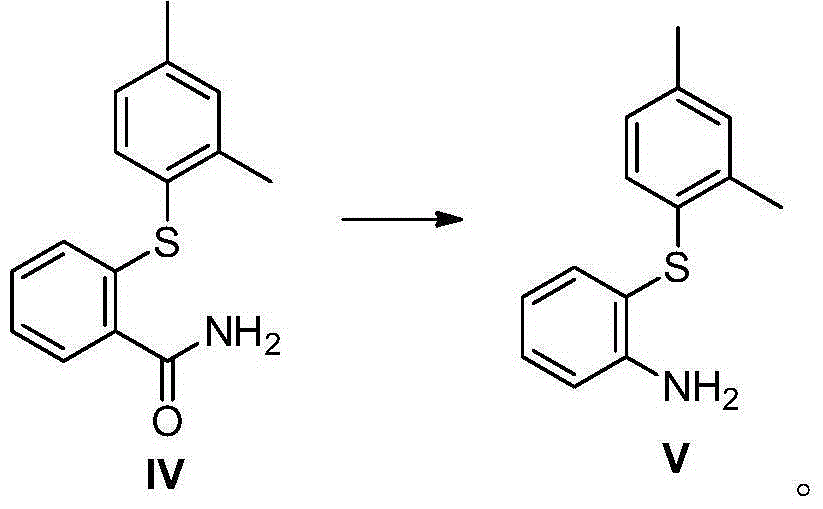
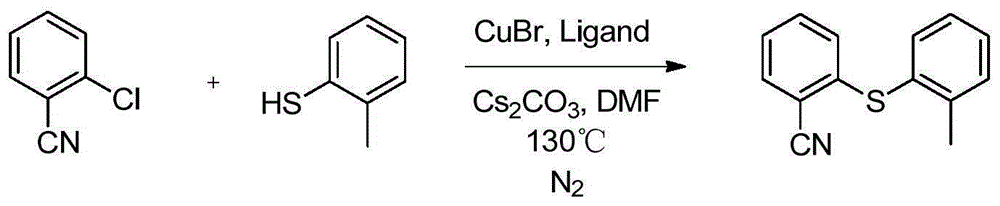Preparation method and intermediate of vortioxetine
A volume and time technology, applied in sulfide preparation, organic chemistry, etc., can solve problems such as danger, environmental pollution, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1 Preparation of intermediate 2-(2,4-dimethylthiophenol) benzonitrile (III) from 2-fluorobenzonitrile (Ia)
[0080]
[0081] Add 2-fluorobenzonitrile (compound as shown in formula Ia) (12.1g, 100mmol), potassium carbonate (16.6g, 120mmol), DMF (100ml) into a 250ml reaction flask, and add 2,4-dimethylbenzene Thiophenol (compound represented by formula II) (14.5 g, 105 mmol) was heated to 100° C. and reacted for 4 hours, and the reaction was completed by TLC dot plate detection. The reaction system was lowered to room temperature, ice water (200ml) was added, and stirred at room temperature for one hour. A white solid was slowly precipitated. The filter cake was washed with 20ml ice water and dried under vacuum at 50°C to obtain 2-(2,4-dimethylthiophenol). ) Benzonitrile (compound as shown in formula III) crude product 24g, yield 100%.
[0082] mp=58.9-60.2℃; MS(m / z): 240[M+H]+; 1HNMR(400MHz, CDCl 3 ,TMS)δ: 7.70(dd,J=8.2,1.2Hz,1H),7.48(d,J=7.8Hz,1H),7.36-7.40(m,1H),7....
Embodiment 2
[0083] Example 2 Preparation of intermediate 2-(2,4-dimethylthiophenol)benzonitrile (III) from 2-chlorobenzonitrile (Ib)
[0084]
[0085] Add 2-chlorobenzonitrile (the compound represented by formula Ib) (13.7g, 100mmol), potassium carbonate (16.6g, 120mmol), DMF (100ml) into the 250ml reaction flask, and then add 2,4-dimethylbenzene Thiophenol (compound represented by formula II) (14.5 g, 105 mmol) was heated to 100° C. and reacted for 4 hours, and the reaction was completed by TLC dot plate detection. The reaction system was lowered to room temperature, ice water (200ml) was added, and stirred at room temperature for one hour. A white solid was slowly precipitated. The filter cake was washed with 20ml ice water and dried under vacuum at 50°C to obtain 2-(2,4-dimethylthiophenol). ) Benzoonitrile (compound as shown in formula III) (23.5g, yield 98%).
[0086] mp=58.9-60.2°C; MS(m / z): 240[M+H] + ; 1 HNMR(400MHz, CDCl 3 ,TMS)δ: 7.70(dd,J=8.2,1.2Hz,1H),7.48(d,J=7.8Hz,1H),7.36-7.40(m...
Embodiment 3a
[0087] Example 3a Preparation of intermediate 2-(2,4-dimethylthiophenol)benzamide (IV)
[0088]
[0089] Add 2-(2,4-dimethylthiophenol)benzonitrile (compound as shown in formula III) (12.0g, 50mmol), potassium hydroxide (11.2g, 200mmol), isopropanol into a 250ml reaction flask (50ml), heated to 80°C and reacted for 2h, the reaction was completed. The reaction system was lowered to room temperature, concentrated under reduced pressure to remove the solvent to obtain a white paste, which was separated by silica gel column chromatography (PE / EA=2 / 1) to obtain a white solid, which was dried under vacuum at 50°C to obtain 12 g of product (as shown in Formula IV) The compound shown), the yield was 93%.
[0090] mp=148.5-150.0℃; MS(m / z): 258[M+H] + ; 1 HNMR(400MHz, CDCl 3 ,TMS)δ: 7.75(dd,J=8.2,1.2Hz,1H), 7.36(d,J=8.0Hz,1H),7.18-7.25(m,3H),7.12(d,J=7.8Hz,1H ), 6.82 (dd, J=8.5, 1.2 Hz, 1H), 6.30 (brs, 2H), 2.42 (s, 3H), 2.35 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com