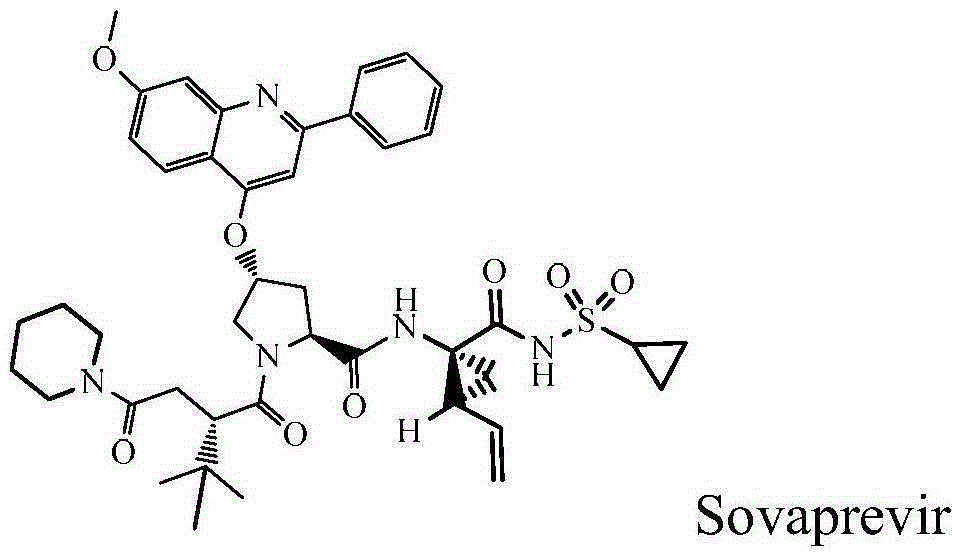
Sovaprevir tablets
A technology of tablets, inhibitors, applied in the field of Sovaprevir tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
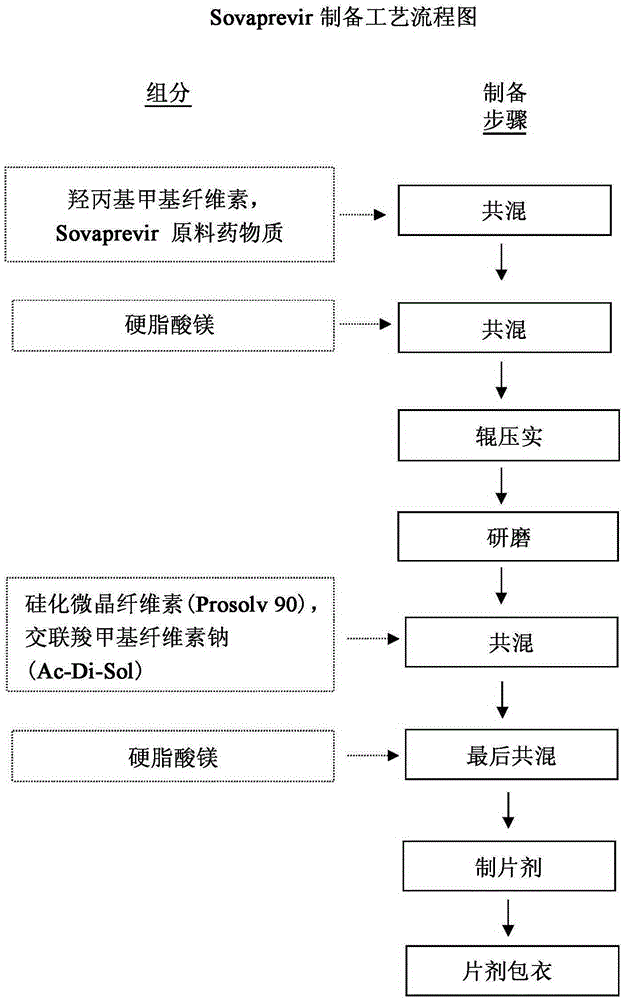
[0045] The manufacturing process of Sovaprevir tablets was optimized by evaluating the blend and tablet physical properties including: bulk and tap density measurements of the blend, flow analysis, sieve analysis, and uniformity; Tablet weight, thickness, hardness, friability, potency, disintegration, dissolution and content consistency testing.
[0046] The present disclosure provides methods for preparing Sovaprevir tablets. In one embodiment, the method includes the following steps.
[0047] First, half of the hydroxypropyl methylcellulose is fed into a blender, such as a V-blender or a box blender, then Sovaprevir is added followed by the remaining half of the hydroxypropyl methylcellulose , and then blend the materials.
[0048] The magnesium stearate can be sieved, such as through a 20 mesh screen, to break up any agglomerates. The sieved magnesium stearate was added to the blender containing the Sovaprevir / crystal growth inhibitor blend and blended for a few minutes....
Embodiment 1
[0061] Example 1. Sovaprevir Tablet Composition
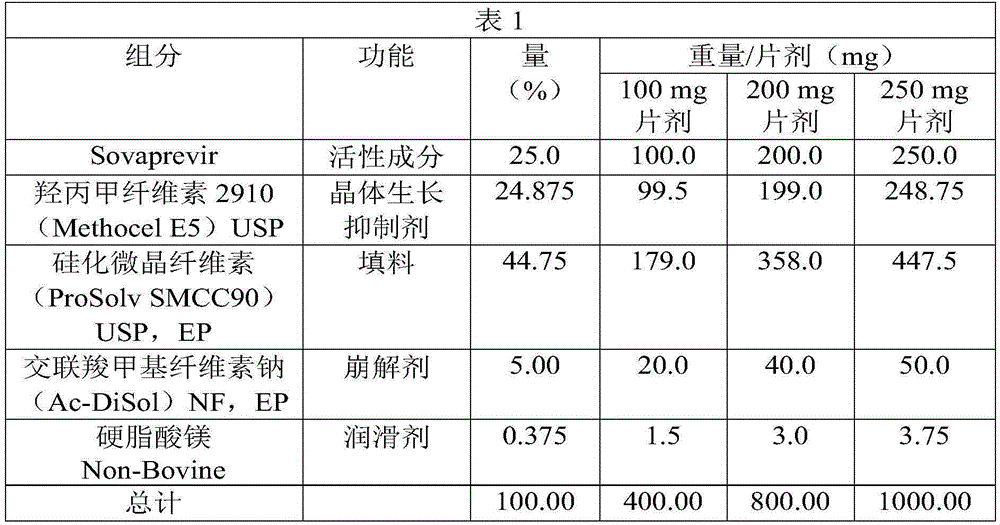
[0062] The three dosage strengths of Sovaprevir tablet cores are for example 100 mg, 200 mg and 250 mg. The 100 mg tablet core size is a standard round concave of 7 / 16 inch and has a theoretical weight of 400 mg. The 200 mg tablet core size is 9 / 16 inch standard circular concave and has a theoretical weight of 800 mg. A 200 mg modified oval tablet core, 0.34 inches by 0.70 inches, is also included. The 250 mg tablet cores are 0.3652 inch x 0.7480 inch modified oval tablet cores with a theoretical weight of 1000 mg. The 300 mg tablet cores were modified oval tablet cores with dimensions 0.3990 inches by 0.7550 inches. All dimensions refer to tooling sizes, actual dimensions of finished tablet cores may vary slightly. All tablet cores were a mottled off-white to yellow appearance. The formulations for the 100mg, 200mg and 250mg tablets are the same. 100, 200 and 250 mg strength tablets were prepared using a common blend by ...
Embodiment 2
[0065] Example 2: Alternative Tablet Formulation
[0066] Another example of tablet formulation is provided in Table 2. The formulations are shown for 100 mg, 200 mg and 250 mg tablets.
[0067]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com