Lansoprazole lipidosome injection
A technology of lansoprazole lipid and lansoprazole, applied in the field of medicine, can solve the problems of low liposome encapsulation rate, difficult to exceed 65%, unstable lansoprazole, etc., and achieves improvement of drug therapeutic index , Improve the stability, reduce the effect of drug side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 Lansoprazole liposome freeze-dried powder injection
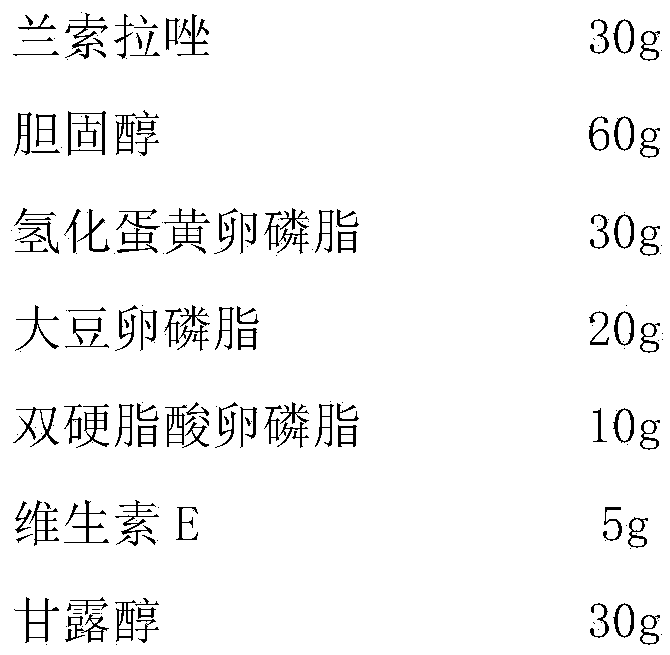
[0035] Prescription (1000 bottles):
[0036]
[0037] Preparation Process:
[0038] 1) Weighing hydrogenated egg yolk lecithin, soybean lecithin, distearate lecithin and cholesterol and antioxidant are dissolved in 1000ml absolute ethanol, heating and melting, heating and steaming ethanol to obtain a phospholipid film;
[0039] 2) Dissolve the phospholipid film in 5000ml of phosphate buffer solution with a pH value of 6.8, add 30g of sterilized lansoprazole and 5g of vitamin E, and 30g of excipient mannitol, stir and dissolve, ultrasonically disperse, filter, and fill in 1000 bottles of vials;
[0040] 3) Pre-freeze at -35°C for 2 hours, then cool down to -45°C for 1 hour, then dry under reduced pressure and vacuum at -10 to -5°C for 10 hours, and finally dry at 35°C for 6 hours , Delansoprazole liposome freeze-dried powder injection.
Embodiment 2
[0041] Embodiment 2 Lansoprazole liposome freeze-dried powder injection
[0042] Prescription (1000 bottles):
[0043]
[0044]
[0045] Preparation Process:
[0046] 1) Weighing hydrogenated egg yolk lecithin, soybean lecithin, distearate lecithin and cholesterol and antioxidant are dissolved in 1000ml absolute ethanol, heating and melting, heating and steaming ethanol to obtain a phospholipid film;
[0047] 2) Dissolve the phospholipid film in 5000ml of pH value 6.8 phosphate buffer solution, add sterilized lansoprazole 30g and vitamin E1g, excipient mannitol 60g, stir and dissolve, ultrasonically disperse, filter, fill in 1000 In a vial;
[0048] 3) Pre-freeze at -30°C for 1 hour, then cool down to -40°C for 1 hour, then dry under reduced pressure and vacuum at -10 to -5°C for 15 hours, and finally dry at 35°C for 5 hours , Delansoprazole liposome freeze-dried powder injection.
Embodiment 3
[0049] Embodiment 3 Lansoprazole liposome freeze-dried powder injection
[0050] Prescription (1000 bottles):
[0051]
[0052] Preparation:
[0053] 1) Weighing hydrogenated egg yolk lecithin, soybean lecithin, distearate lecithin and cholesterol and antioxidant are dissolved in 1000ml absolute ethanol, heating and melting, heating and steaming ethanol to obtain a phospholipid film;
[0054] 2) Dissolve the phospholipid film in 5000ml of phosphate buffer solution with a pH value of 6.8, add 30g of sterilized lansoprazole, 5g of vitamin E, and 60g of mannitol, stir and dissolve, ultrasonically disperse, filter, and fill in 1000 vials middle;
[0055] 3) Pre-freeze at -35°C for 2 hours, then cool down to -40°C for 1 hour, then dry under reduced pressure and vacuum at -10 to -5°C for 10 hours, and finally dry at 35°C for 5 hours , Delansoprazole liposome freeze-dried powder injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com