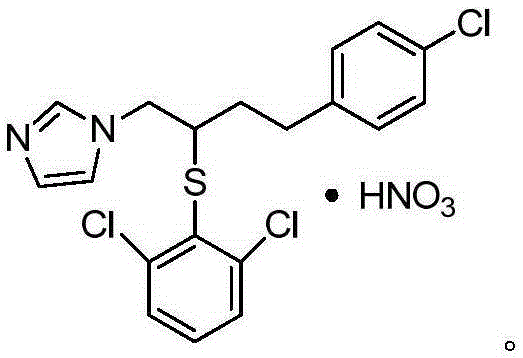
Industrial production method for butoconazole nitrate intermediate
A technology of butoconazole nitrate and intermediates, which is applied in the field of production of butoconazole nitrate intermediate 1-chloro-4--2-butanol, can solve the problem that the product yield and purity cannot meet industrial production, butoconazole nitrate Solve the problems of complex azole production process and high cost, and achieve the effect of being suitable for large-scale industrial production, reasonable raw materials and dosage, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
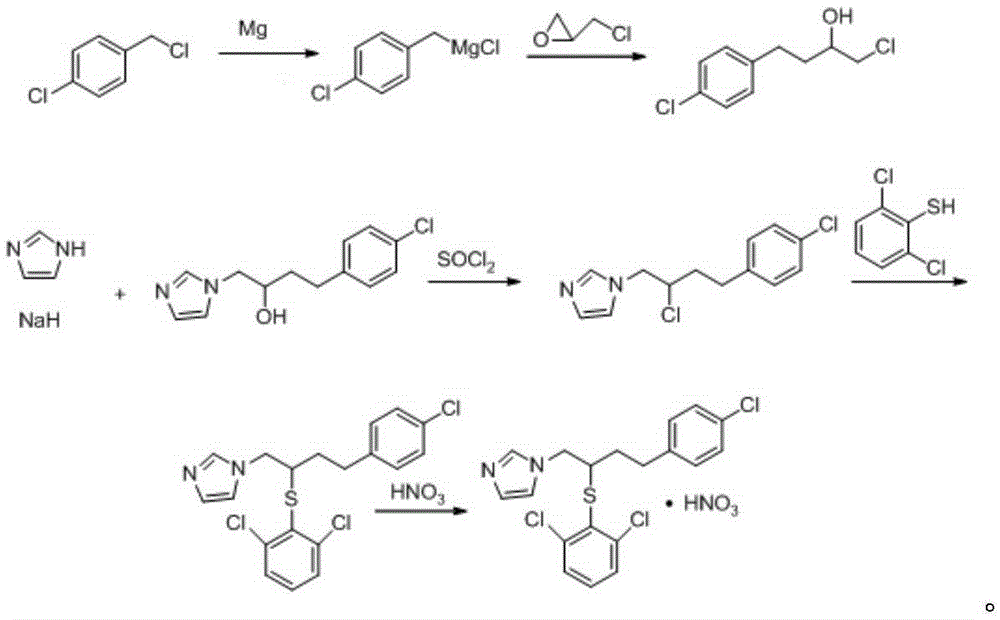
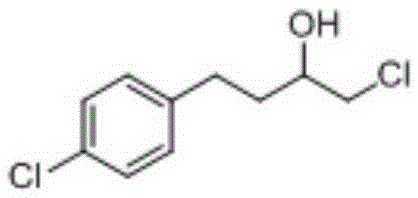
[0036] Prepare butoconazole nitrate intermediate 1-chloro-4-(4-chlorophenyl)-2-butanol as follows:
[0037] (1) Get 2kg of magnesium powder, 50ml of ether and a catalytic amount of iodine, add 40kg of p-chlorobenzyl ether solution with a concentration of 0.27g / ml dropwise at a rate of 3ml / s at 35°C, and after the dripping, place the solution at 37°C React for 1 hour to obtain p-chlorobenzyl Grignard reagent; then slowly add 20 kg of epichlorohydrin ether solution with a concentration of 0.35 g / ml dropwise, and react at 37° C. for 1.5 hours after the drop to obtain a reaction solution;
[0038] (2) Take the reaction solution obtained in step (1), add the sulfuric acid with a concentration of 25% dropwise at a rate of 2ml / s at 4°C under constant stirring, and stop the dripping when there is no solid residue; leave standstill after stirring at a constant speed for 10min The liquid was separated for 10 minutes; the aqueous phase was discarded, and the organic phase was concentrate...
Embodiment 2
[0041] Compared with Example 1, the difference is only that the step (1) is specifically:
[0042] (1) Get 1.5kg of magnesium powder, 50ml of ether and a catalytic amount of iodine, add 35kg of p-chlorobenzyl ether solution with a concentration of 0.25g / ml dropwise at a rate of 2ml / s at 30°C, The mixture was reacted for 0.5 hour to obtain the p-chlorobenzyl Grignard reagent; then slowly added dropwise 15 kg of epichlorohydrin ether solution with a concentration of 0.33 g / ml, and reacted at 35° C. for 1 hour to obtain the reaction solution.
[0043] The yield of the product of this embodiment is 78.1%.
[0044] After testing, the content of the target product 1-chloro-4-(4-chlorophenyl)-2-butanol is 90.11%, the content of p-chlorotoluene is 7.51%, and the content of chloromethylbenzene is 0.20%, 1, The content of 2-(4-chlorophenyl)ethane was 1.63%, the content of 1-chloro-4-(4-(chloro)benzyl)benzene was 0.19%, the content of 3-chloro-2-(4-chlorobenzyl base)-1-propanol content...
Embodiment 3
[0046] Compared with Example 1, the difference is only that the step (1) is specifically:
[0047] (1) Get 2.5kg of magnesium powder, 50ml of ether and catalytic amount of iodine, add 45kg of p-chlorobenzyl ether solution with a concentration of 0.3g / ml dropwise at a rate of 4ml / s at 40°C, The mixture was reacted for 1.5 hours to obtain the p-chlorobenzyl Grignard reagent; then slowly added dropwise 25 kg of epichlorohydrin ether solution with a concentration of 0.37 g / ml, and reacted at 40° C. for 2 hours to obtain the reaction solution.
[0048] The yield of the product of this embodiment is 75.8%.
[0049] After testing, the content of the target product 1-chloro-4-(4-chlorophenyl)-2-butanol is 83.12%, the content of p-chlorotoluene is 12.33%, and the content of chloromethylbenzene is 0.55%, 1, The content of 2-(4-chlorophenyl)ethane is 2.31%, the content of 1-chloro-4-(4-(chloro)benzyl)benzene is 0.41%, and the content of 3-chloro-2-(4-chlorobenzyl) The content of 1-prop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com