Compound, preparation method and antibacterial application thereof
A technology of compounds and bacteriostats, applied in the field of compounds and their preparation, to achieve the effect of less raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
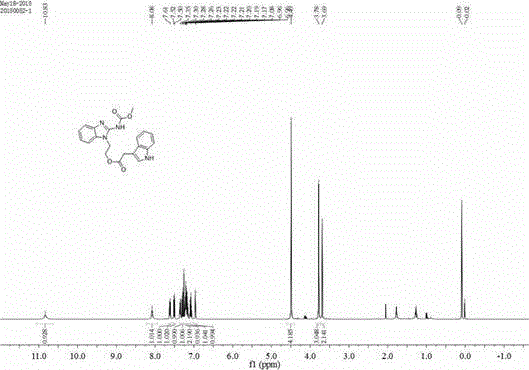
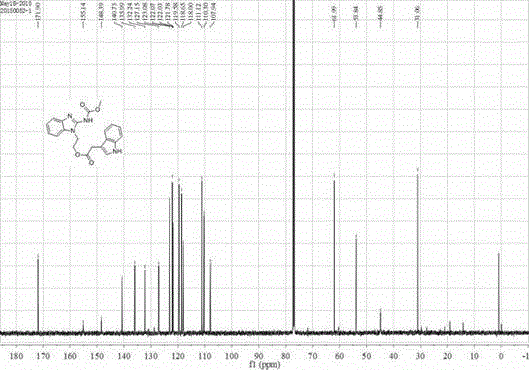
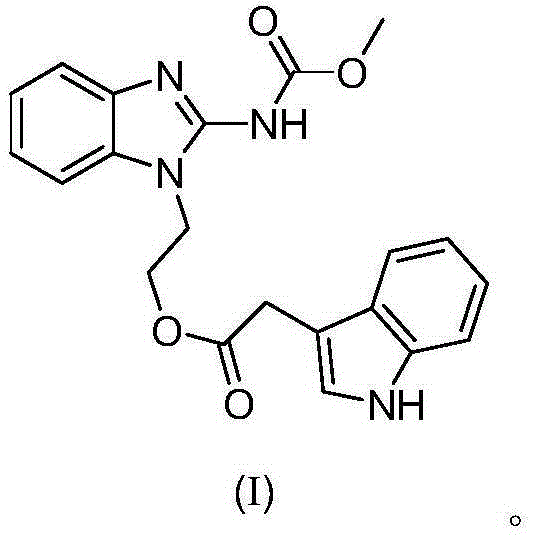
[0036] The preparation of embodiment 1 formula (I) compound
[0037]
[0038] Add 10 mmol of indole acetic acid (II), 10 mmol of N,N'-dicyclohexylcarbodiimide (DCC), a catalytic amount of 4-dimethylaminopyridine (DMAP) and 12 mmol of bromoethanol (III) to 30 mL of dichloromethane , stirred at room temperature for 2h. After completion of the reaction, add a large amount of water to extract, concentrate, and column chromatography to obtain a colorless intermediate compound (IV).
[0039]
[0040] Add 5mmol of carbendazim (V) to 20mL of dimethylformamide, stir at room temperature, add 7.5mmol of cesium carbonate, stir for 30min, cool down to 0°C, and dropwise add 6mmol of intermediate compound (IV) in N , N-dimethylformamide (DMF) solution 30mL, slowly warming up to room temperature, stirring for 36h. After the reaction was completed, the pH value was adjusted to neutral with 3mol / L HCl solution, extracted with ethyl acetate, and column chromatography was used to obtain t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com