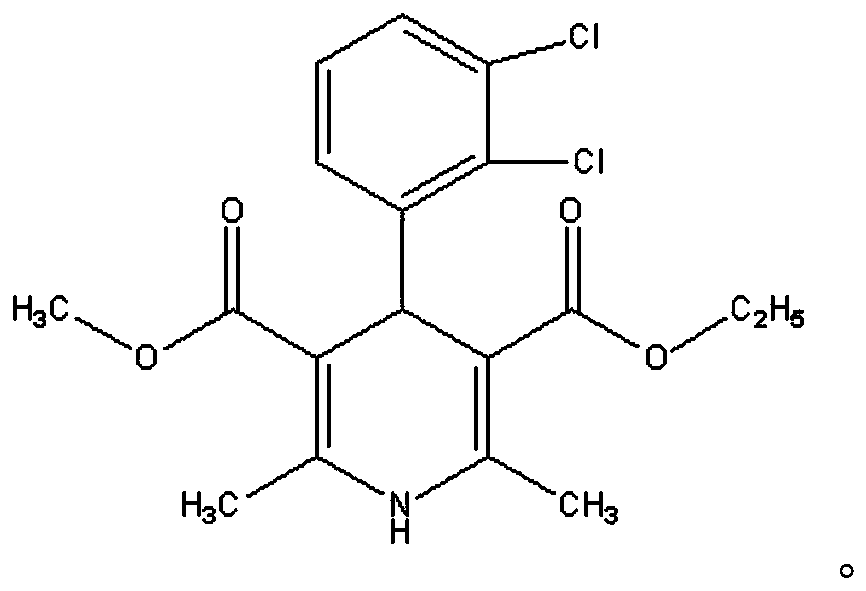
A kind of felodipine sustained-release tablet and preparation method thereof
A felodipine and gentle technology, applied in the direction of non-active ingredient medical preparations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problem of large blood pressure fluctuations, frequent medications, and restrictions on the development of felodipine sustained-release tablets and other problems, to achieve the effect of improving uniformity, good sustained release effect, sustained release effect and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention also provides a preparation method of the felodipine sustained-release tablet described in the above technical scheme, comprising the following steps:
[0044] A) cooling after mixing felodipine with the dispersed dispersion matrix after melting, obtains felodipine solid dispersion; Described dispersion matrix is polyethylene glycol;
[0045] b) compressing the felodipine solid dispersion, filler, lubricant and slow-release material after mixing to obtain a felodipine sustained-release tablet; the slow-release material is hydroxypropyl methylcellulose.
[0046] In the present invention, felodipine is mixed with the molten dispersion matrix and then cooled to obtain a felodipine solid dispersion. In the present invention, the felodipine is the main ingredient; the source of the felodipine is not particularly limited in the present invention, and commercially available products well known to those skilled in the art can be used. In the present inv...
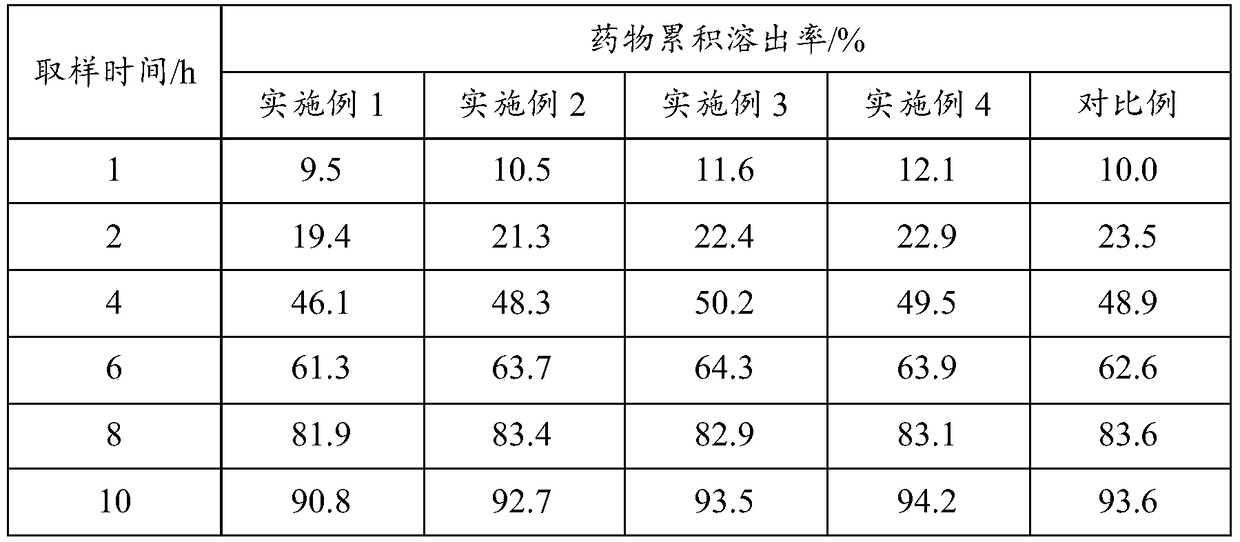
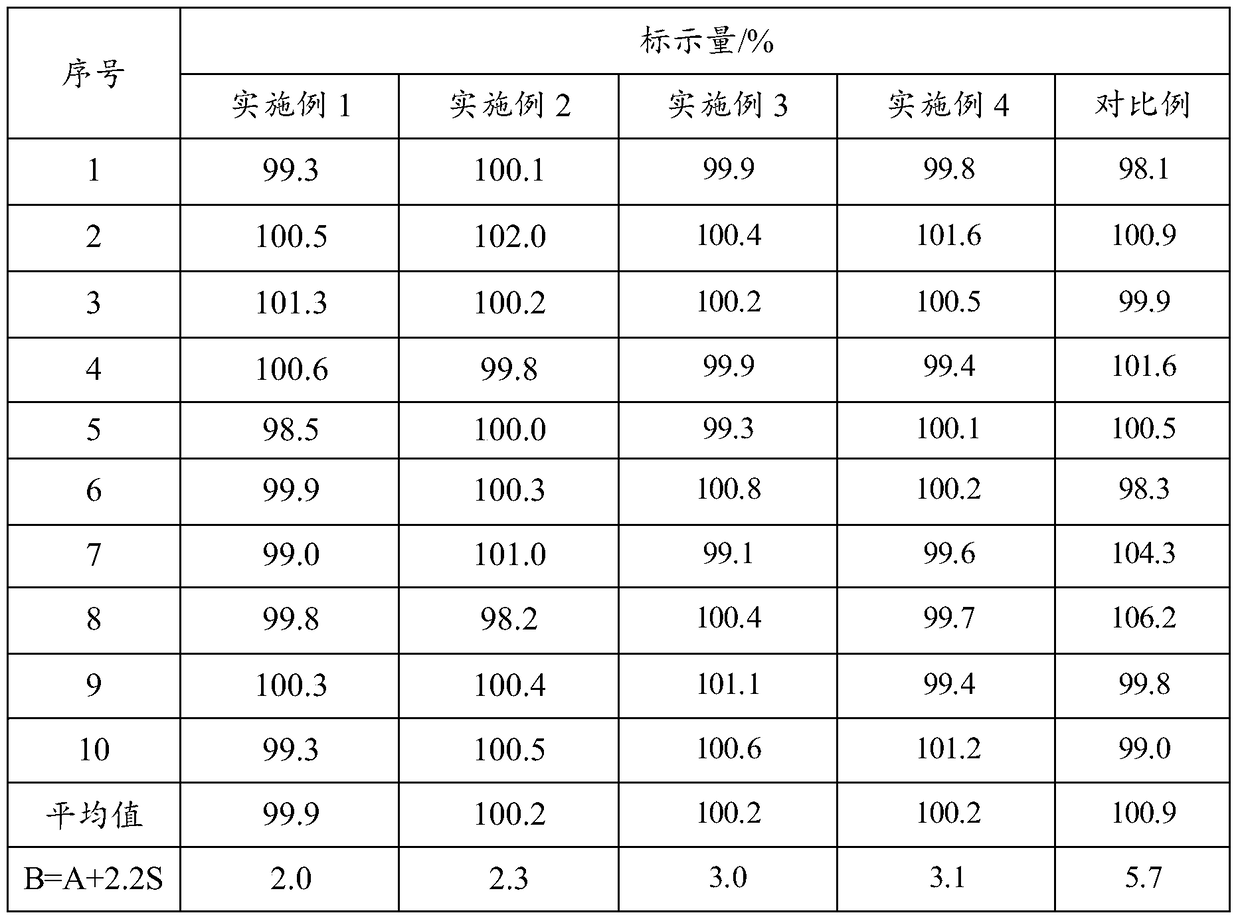
Embodiment 1
[0067] (1) Heat 50g of polyethylene glycol 6000 in a water bath at 70°C until it is completely melted, then add 5g of felodipine crude drug and mix, then cool in an ice bath for 12 hours to obtain a completely embrittled solid product, and finally pulverize Cross 80 mesh sieves to obtain felodipine solid dispersion.
[0068](2) Combine the obtained felodipine solid dispersion with 48g lactose, 12g mannitol, 20g microcrystalline cellulose, 2g micropowder silica gel, 4g magnesium stearate, 21g hydroxypropyl methylcellulose K250 and 42g hydroxypropyl methylcellulose Methylcellulose K750 is added in the mixer, stirred at 4r / min for 30min, finally adopts tablet press to carry out tabletting, tabletting parameter is φ 9mm, hardness 6kg, obtains felodipine slow-release tablet (1000).
[0069] (3) Adopt 25g Opadry coating powder to carry out coating, obtain felodipine sustained-release tablet product.
Embodiment 2
[0071] (1) Heat 30g of polyethylene glycol 10000 in a water bath at 70°C until it is completely melted, then add 5g of felodipine bulk drug and mix, then cool in an ice bath for 12 hours to obtain a completely embrittled solid product, and finally pulverize Cross 80 mesh sieves to obtain felodipine solid dispersion.
[0072] (2) Felodipine solid dispersion obtained with 68g lactose, 32g microcrystalline cellulose, 2g micropowder silica gel, 4g magnesium stearate, 18g hydroxypropyl methylcellulose K250 and 45g hydroxypropyl methylcellulose K750 is added in the mixer, stirred at 3r / min for 30min, finally adopts tablet press to carry out tabletting, tabletting parameter is φ 9mm, hardness 6kg, obtains felodipine slow-release tablet (1000).
[0073] (3) Adopt 25g Opadry coating powder to carry out coating, obtain felodipine sustained-release tablet product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com