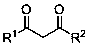
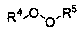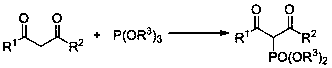Method for preparing 2-phosphonic acid ester base-1, 3-dicarbonyl derivative
A technology of phosphonate-based and derivatives, which is applied in the field of preparation of organic compounds, can solve the problems of heavy pollution and difficulty in obtaining raw materials, and achieve the effects of low pollution, short reaction time, and favorable production safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of 2-dimethylphosphonate-1,3-diphenyl-1,3-propanedione
[0038] Using 1,3-diphenyl-1,3-propanedione and trimethyl phosphite as raw materials, the reaction steps are as follows:
[0039] Add 1,3-diphenyl-1,3-propanedione (0.224g, 1.0mmol), trimethylphosphite (0.124g, 1.0mmol), cuprous chloride (0.010g, 0.1mmol), tert-butyl hydroperoxide (0.09g, 1mmol), and ethanol (5ml), react at 70°C;
[0040] TLC tracking reaction until complete completion;
[0041] The crude product obtained after the reaction was separated by column chromatography (petroleum ether:ethyl acetate=4:1) to obtain the target product (yield 54%). The analytical data of the product are as follows: 1 HNMR (400MHz, DMSO-d 6 ):δ8.09(d,J=7.4Hz,4H),7.71(t,J=7.4Hz,2H),7.58(t,J=7.7Hz,4H),7.17(d,1H),3.71(d ,6H).
Embodiment 2
[0042] Example 2: Synthesis of 2-dimethylphosphonate-1-(4-methylphenyl)-3-phenyl-1,3-propanedione
[0043] Using 1-(4-methylphenyl)-3-phenyl-1,3-propanedione and trimethylphosphite as raw materials, the reaction steps are as follows:
[0044] Add 1-(4-methylphenyl)-3-phenyl-1,3-propanedione (0.238g, 1.0mmol), trimethylphosphite (0.248g, 2.0mmol), Cuprous chloride (0.010g, 0.1mmol), tert-butyl hydroperoxide (0.18g, 2mmol) and acetonitrile (5ml), react at 70°C;
[0045] TLC tracking reaction until complete completion;
[0046] The crude product obtained after the reaction was separated by column chromatography (petroleum ether:ethyl acetate=4:1) to obtain the target product (yield 64%). The analytical data of the product are as follows: 1 HNMR (400MHz, DMSO-d 6 ):δ8.03(dd,J=25.2,7.9Hz,4H),7.70(s,1H),7.57(d,J=7.7Hz,2H),7.38(d,J=8.0Hz,2H),3.84 –3.64 (m, 6H), 2.49 – 2.33 (m, 3H).
Embodiment 3
[0047] Example 3: Synthesis of 2-dimethylphosphonate-1,3-bis(4-methylphenyl)-1,3-propanedione
[0048] Using 1,3-bis(4-methylphenyl)-1,3-propanedione and trimethylphosphite as raw materials, the reaction steps are as follows:
[0049] Add 1,3-bis(4-methylphenyl)-1,3-propanedione (0.252g, 1.0mmol), trimethylphosphite (0.372g, 3.0mmol), chloride Copper (0.013g, 0.1mmol), tert-butyl hydroperoxide (0.27g, 3mmol) and acetic acid (5ml) were reacted at 70°C;
[0050] TLC tracking reaction until complete completion;
[0051] The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the target product (yield 90%). The analytical data of the product are as follows: 1 HNMR (400MHz, DMSO-d 6 ):δ8.05(d,J=7.9Hz,4H),7.08(d,J=7.9Hz,4H),6.98(d,J=7.8Hz,1H),3.87(s,6H),3.71(d , J=11.2Hz, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com