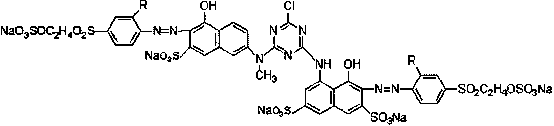
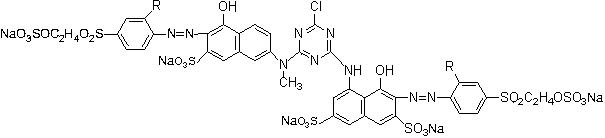
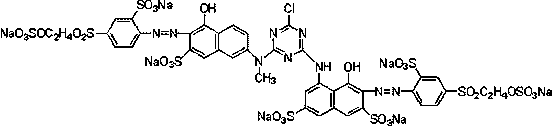
Bright red reactive dye and preparation method and application thereof
A reactive dye and red technology, applied in reactive dyes, dyeing methods, azo dyes, etc., can solve the problems that reactive dyes cannot meet the requirements of beauty lovers, achieve excellent wet rubbing fastness, improve level dyeing, and save production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Its preparation method comprises the following steps:
[0034] a, condensation reaction: N-methyl J acid is dissolved with liquid caustic soda to obtain N-methyl J acid solution, N-methyl J acid solution is added in cyanuric chloride to react to obtain a condensation liquid, then add H acid aqueous solution to obtain a second secondary condensation liquid;
[0035] b. Diazotization reaction: add hydrochloric acid to para-ester or sulfonated para-ester first, and then add sodium nitrite to react to obtain para-ester diazonium solution or sulfonated para-ester diazonium solution;
[0036] c. Coupling reaction: adding the para-ester diazo solution or sulfonated para-ester diazo solution obtained in step b into the secondary condensation solution obtained in step a to obtain a coupling reaction solution;
[0037] d. Drying: drying the coupling reaction solution obtained in step c to obtain the compound of formula I.
Embodiment 1
[0040] Grind 100 parts of cyanuric chloride on ice for 30 minutes, and control the temperature T=2°C; dissolve 100 parts of N-methyl J acid with liquid alkali, adjust the pH=7.0, add it to cyanuric chloride, and use a small Dry soda powder to adjust pH=3.5, react for 2 hours, HPLC detects that N-methyl J acid disappears, and obtains a primary condensation liquid;
[0041] Dissolve 100 parts of H acid with an appropriate amount of water, adjust the pH to 6.5 with liquid caustic soda, add it to the primary condensation liquid, adjust the pH to 5.0 with dry baking soda, and raise the temperature to 45°C at the same time, react for 5 hours, and detect the disappearance of H acid by HPLC. Obtain the secondary condensation liquid (dicondensate), and cool down to 12°C for use;
[0042] Add 200 parts of para-ester to an appropriate amount of water for beating for 1 hour, add hydrochloric acid (240 parts in terms of hydrogen chloride), cool down to T=3°C, add 205 parts of sodium nitrit...
Embodiment 2
[0047] Grind 100 parts of cyanuric chloride on ice for 30 minutes, control the temperature T=3°C; dissolve 98 parts of N-methyl J acid with liquid alkali, adjust the pH=6.5, add it to cyanuric chloride, and use a small Adjust the pH to 3.5 with dry soda powder, react for 2 hours, and detect the disappearance of N-methyl J acid by HPLC to obtain a primary condensation solution.
[0048] Dissolve 98 parts of H acid with an appropriate amount of water, adjust the pH to 6.5 with liquid caustic soda, add it to the primary condensation liquid, adjust the pH to 5.0 with baking soda dry powder, and raise the temperature to 40°C at the same time, react for 6 hours, and detect the disappearance of H acid by HPLC. The secondary condensation solution was obtained, cooled to 12°C, and set aside.
[0049] Add 205 parts of para-ester with appropriate amount of water and beat for 1 hour, add hydrochloric acid (240 parts in terms of hydrogen chloride), cool down to T=0°C, add 209 parts of sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com