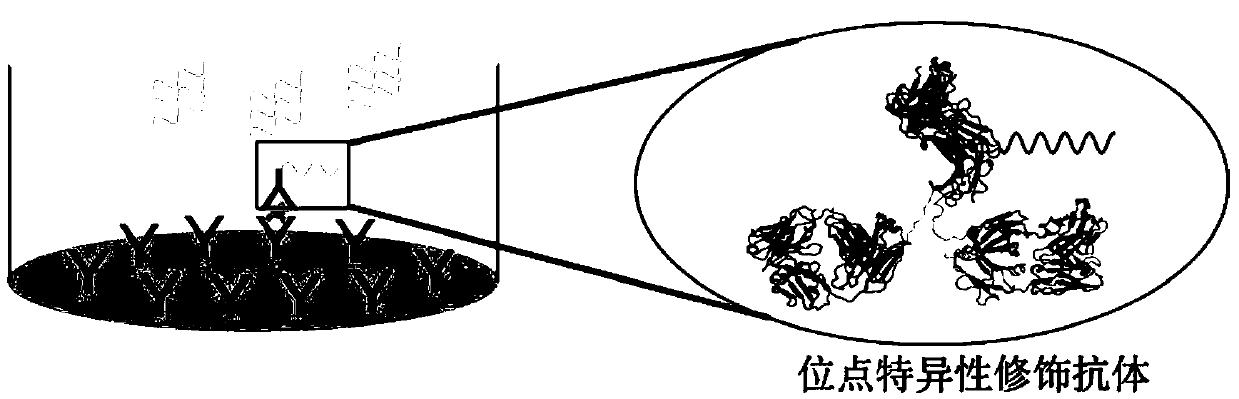
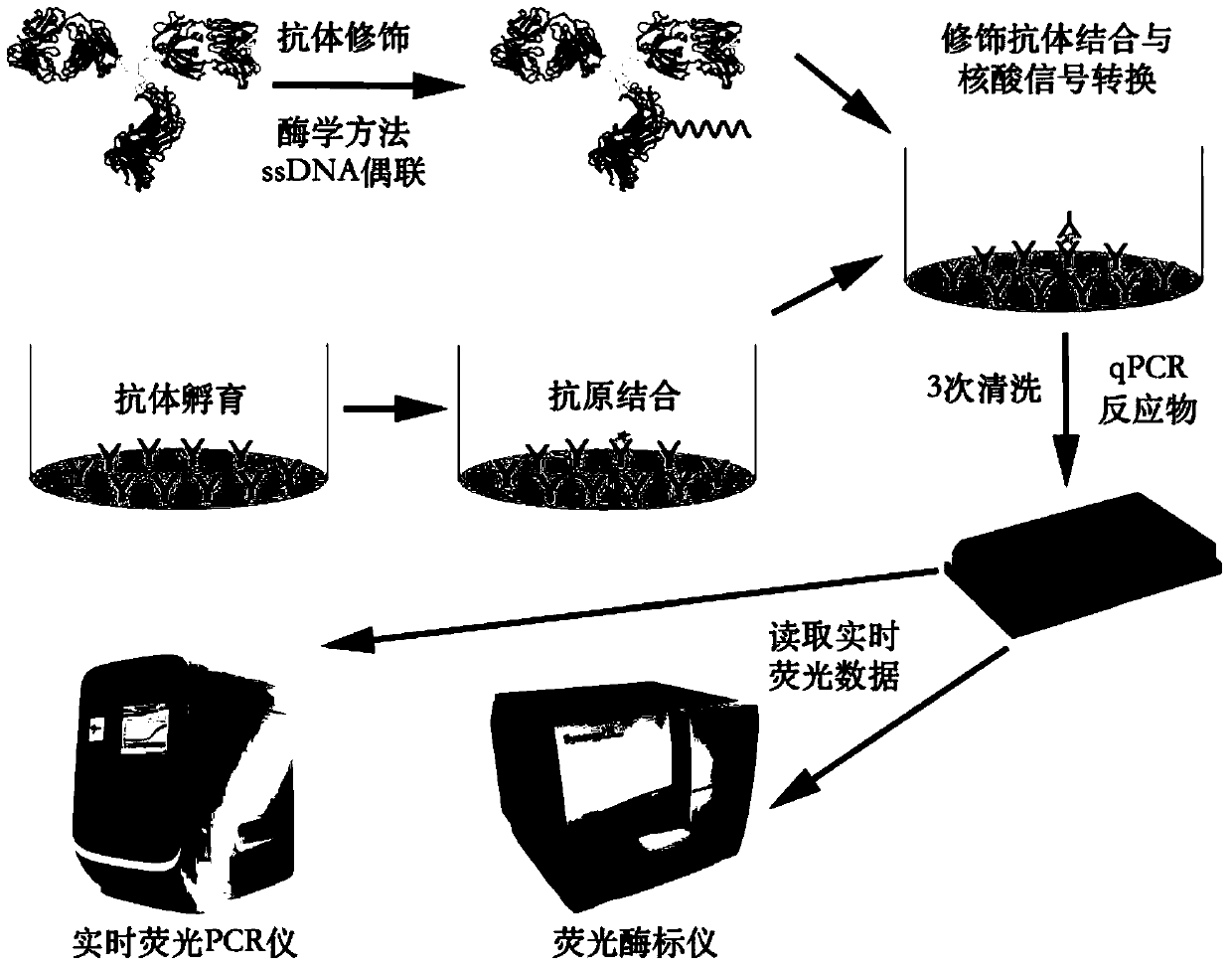
Detection kit and detection method using antibody to modify immuno-PCR reaction
A detection kit and antibody modification technology, which is applied in the field of immunological detection, can solve the problems of varying degrees of improvement, difficulty in breaking through 1000 times of sensitivity, and decline in antibody specificity and affinity, achieving a wide dynamic range, wide detection sensitivity, and simplified The effectiveness of the detection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Biotin Modification of Insulin Antibody 3A6
[0069] The Insulin monoclonal antibody 3A6 (ab1965) from Abcam needs to be modified with biotin before it can be specifically coated into a streptavidin PCR96well microplate (Pierce, CatNo.15500) for Immuno-PCR detection . The antibody was modified using the SiteClick series labeling kit (S10467) and DIBO-labeled biotin (C10412) from LifeTechnologies.
[0070] (1) Purification and modification of antibodies are carried out first.
[0071] The first round of purification: first add 0.5mg / ml 250μL 3A6 antibody solution to the antibody concentrator (an ultrafiltration centrifuge tube with unknown molecular weight cut-off) attached to the kit, which can concentrate the antibody and replace the buffer; Then add 500 μL of antibody replacement buffer (bufferA) to the concentrator and centrifuge at 6000×g at 4°C for 5 minutes. After the centrifugation is completed, use a pipette to suck up the remaining solution to wash...
Embodiment 2
[0076] Example 2: ssDNA modification of Insulin antibody
[0077] (1) Synthesis of ssDNA with DIBO marker (dibenzocyclooctyne):
[0078] a. Generate a random sequence with a length of 100bp through the DNA sequence random synthesis website (http: / / www.facμLty.ucr.edu / ~mmaduro / random.htm)
[0079] 5'-ATGGGGCTGGATAAAACTGCCCTGGTGACCGCCATCAACAACCCGAATACGTGGCATTTCAGGAGGCGGCCGGAGGGGGATGTTTTCTACTATTCGAGG-3' (SEQ ID No. 1).
[0080] Through the blastn search program (http: / / www.ncbi.nlm.nih.gov / BLAST / Blast.cgi), it was confirmed that the sequence does not have any known sequences homologous to it, so as to ensure that the subsequent PCR detection signal is specific information from antibody binding .
[0081] After determining the sequence of the ssDNA, the 5' terminal DIBO marked ssDNA (SEQ ID No.1) and two PCR primers for amplifying the fragment were synthesized by IDT (www.idtdna.com):
[0082] 5'-ATGGGGCTGGATAAAACTGC-3' (SEQ ID No.2) and
[0083] 5'-CCTCGAATAGTAGAAAACATCCCC-3'...
Embodiment 3
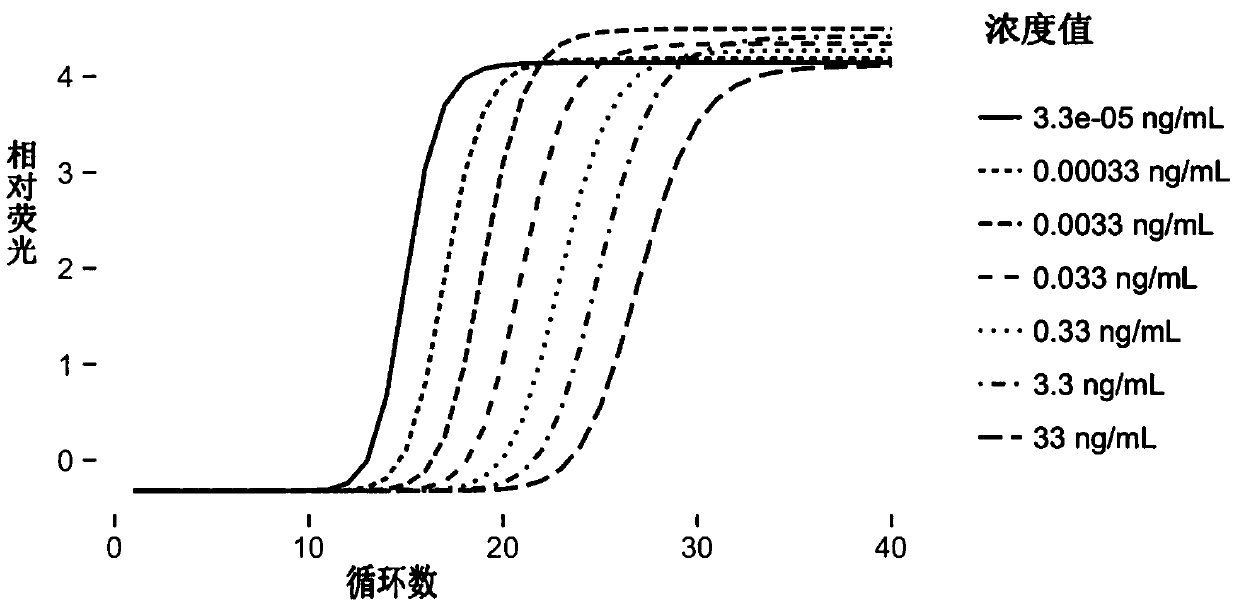
[0091] Embodiment 3: the making of Insulin immune PCR standard curve
[0092] (1), Preparation of Immuno-PCR microwell plate:
[0093] The DIBO-labeled biotin-modified Insulin antibody 3A6 solution obtained in Example 1 was coated on a streptavidin PCR microwell plate (Pierce Company, CatNo. 15500): after overnight incubation for 12 hours at 4°C, Antibody-coated microwell plates were washed three times with PBST buffer (1×PBS, 0.1% Tween20), and then incubated with PBST-BSA buffer (1×PBS, 0.1% Tween20, 5% BSA) at 4°C The plate was left overnight, and then the microplate was washed with PBST buffer three times for use to obtain an Immuno-PCR microplate.
[0094] (2), InsuLin Immuno-PCR standard curve formulation:
[0095] a. First prepare the human source Insulin standard substance (mother solution, 10mg / mL, Sigma Company), the concentration of the standard substance is from 33ng / ml to 3.3x10E-5ng / ml10-fold equal dilution, and there are 7 different concentrations in total; th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com