Medicine composition capable of alleviating asthenopia and preparation method of medicine composition
A technology for relieving visual fatigue and composition, which is applied in the directions of drug combination, pharmaceutical formula, active ingredient of hydroxyl compound, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method of a pharmaceutical composition for alleviating asthenopia, comprising:
[0031] 1) Weigh the raw materials according to the following parts by mass: 280-320g of Ginkgo biloba, 18-22g of bilberry extract, 230-270g of medlar, 0-20g of Ligustrum lucidum, 0-15g of Polygonum multiflorum, 280-320g of chrysanthemum, cassia seed 0-35g, 8-10g taurine, 9.3-9.7g zinc gluconate, 40-60mg vitamin A; among them, the vitamin A raw material with a purity of 10% is used, that is, the raw material of vitamin A with a purity of 10% is weighed 400~600mg;
[0032] 2) Ginkgo biloba leaves, wolfberry fruit, privet privet fruit, Polygonum multiflorum, chrysanthemum, and cassia seeds are placed in a multi-functional extraction tank, added with 8 times the amount of drinking water, soaked for 20 minutes, steam heated to 100°C, decocted for 1.5 hours, poured Take out the decoction, filter it with a 100-mesh screen to obtain the filtrate (I); decoct the dregs with 6 times the...
Embodiment 2
[0039] The difference between embodiment 2 and embodiment 1 is: in the described step 1), the crude drug is weighed in the following parts by mass: Ginkgo biloba 300g, bilberry extract 20g, medlar 250g, chrysanthemum 300g, taurine 9g, zinc gluconate 9.5g, vitamin A 50mg; Wherein, vitamin A adopts the vitamin A raw material that purity is 10%, namely takes by weighing 500mg of vitamin A raw material that this purity is 10%; All the other steps are with embodiment 1.
[0040] In this example, every 100 g of the pharmaceutical composition contains 0.5 g of anthocyanins, 16 mg of vitamin A, 2.4 g of taurine, and 210 mg of zinc.
Embodiment 3
[0042] The difference between embodiment 3 and embodiment 1 is that: in the step 1), the crude drug is weighed in the following parts by mass: 300g of ginkgo biloba, 20g of bilberry extract, 2350g of medlar, 5g of privet fruit, Polygonum multiflorum 5g, chrysanthemum 300g, cassia seed 10g, taurine 9g, zinc gluconate 9.5g, vitamin A 50mg; Wherein, vitamin A adopts the raw material of vitamin A that is 10% purity, promptly takes this purity and is 500mg of vitamin A raw material of 10%; All the other steps are the same as in Example 1.
[0043] In this example, every 100 g of the pharmaceutical composition contains 0.5 g of anthocyanins, 16 mg of vitamin A, 2.4 g of taurine, and 210 mg of zinc.
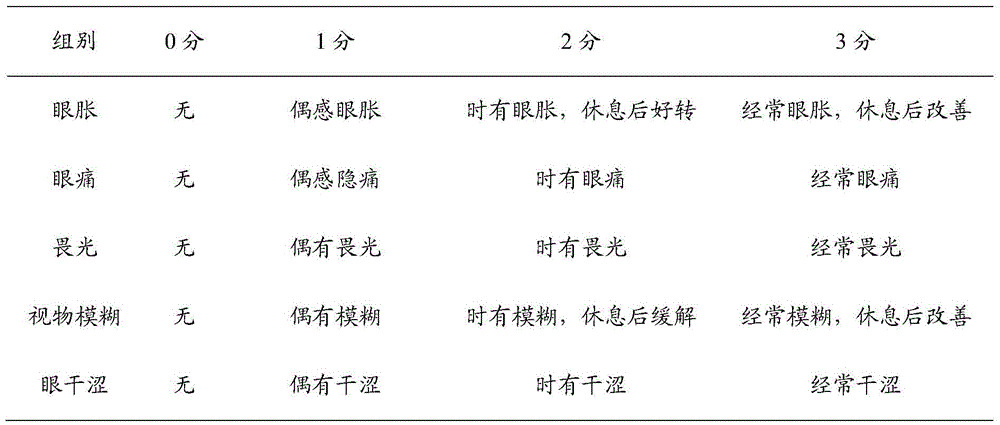
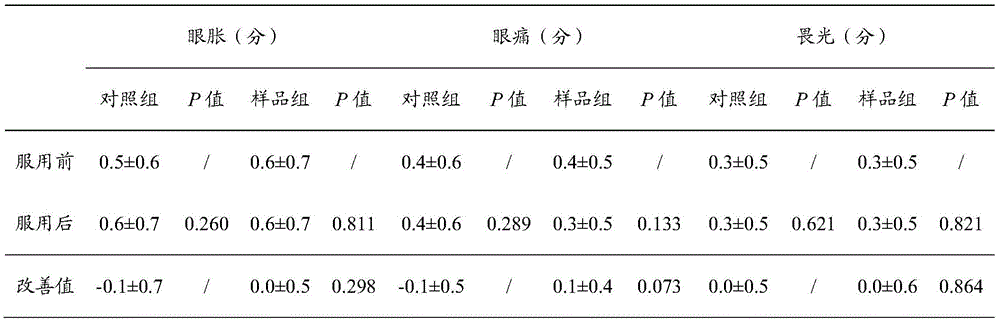
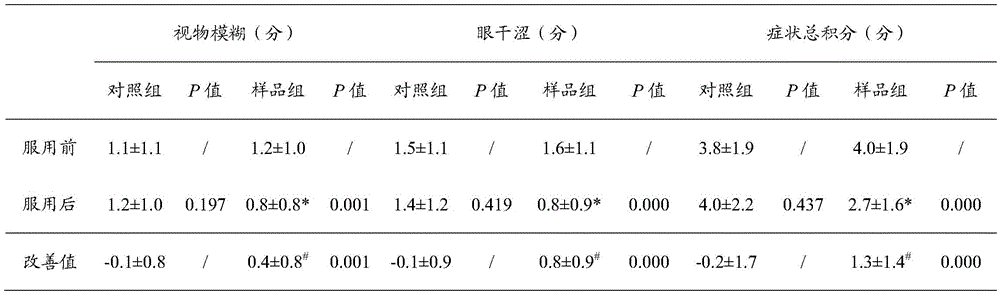
[0044] Those skilled in the art will know that when the technical parameters of the present invention change within the following ranges, the same or similar effects as those of the above-mentioned embodiments can be expected:
[0045] A pharmaceutical composition for relieving asthenopi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com