Arctigenin amino-acid ester derivatives, and preparation method and application thereof
A technology of arctigenin and amino acids, which is applied in neurodegenerative disease drugs, new arctigenin amino acid ester derivatives and its preparation field, and can solve the problem of high plasma protein binding rate, low pass rate, and low bioavailability of blood-brain Barrier and other issues, to achieve the effect of easy synthesis, reasonable design, and significant neuroprotective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
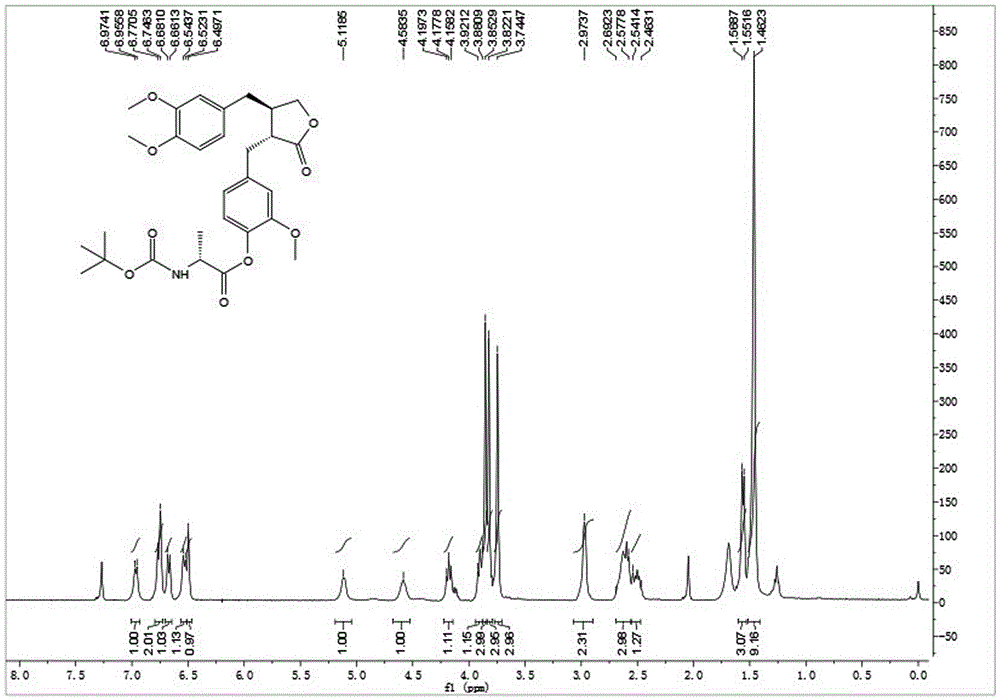
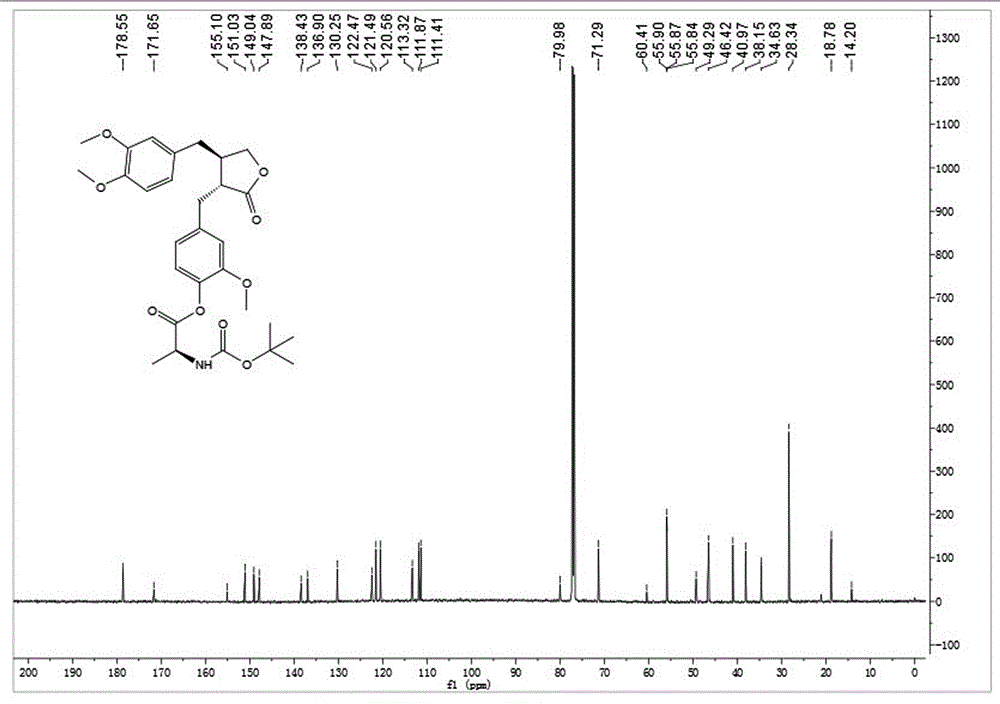
[0037] Embodiment 1: synthetic compound 1-1, please refer to figure 1 and figure 2
[0038] .
[0039] Dissolve N-Boc-L-alanine (30.4 mg, 0.16 mmol) in dry toluene, add 2,4,6-trichlorobenzoyl chloride (30.5 μL, 0.20 mmol) and DIPEA (26.0 μL, 0.20 mmol ), after stirring for 10 minutes, arctigenin (50mg, 0.134mmol) was added, DMAP (32.7mg, 0.27mmol) was added at one time, and stirring was continued at room temperature for 3h, TLC showed that the reaction was almost complete. After concentration under reduced pressure, silica gel thin-layer preparation plate was purified to obtain 63 mg of white powder with a yield of 86%. [α] D 25 =-25.6(c1.0, CHCl3 ); 1 HNMR (400MHz, CDCl 3 )δ6.96(d,J=7.3Hz,1H),6.76(d,J=9.7Hz,2H),6.67(d,J=7.9Hz,1H),6.53(d,J=8.2Hz,1H) ,6.50(s,1H),5.12(m,1H),4.58(m,1H),4.18(t,J=7.8Hz,1H),3.93–3.88(m,1H),3.85(s,3H), 3.82(s,3H),3.74(s,3H),2.97(s,2H),2.68–2.56(m,3H),2.51(m,1H),1.56(d,J=6.8Hz,3H),1.46 (s,9H);ESI-MS(m / z):[M+Na] + =566.3(Calcd:523.2).
Embodiment 2
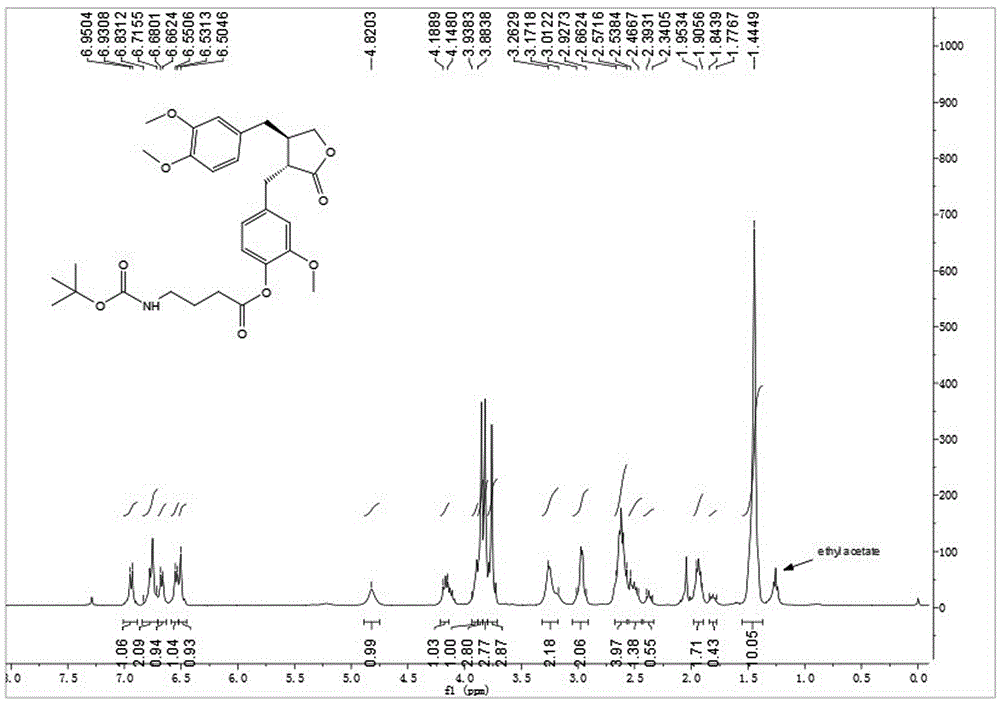
[0040] Embodiment 2: synthetic compound 1-2, please refer to image 3 and Figure 4
[0041] .
[0042] Dissolve N-Boc-GAMMA-aminobutyric acid (32.5 mg, 0.16 mmol) with CAS No. 57294-38-9 in dry toluene, add 2,4,6-trichlorobenzoyl chloride (30.5 μL, 0.20 mmol ) and DIPEA (26.0 μL, 0.20 mmol), after stirring for 10 min, arctigenin (50 mg, 0.134 mmol) was added, and DMAP (32.7 mg, 0.27 mmol) was added at one time, and stirring was continued at room temperature for 3 h. TLC showed that the reaction was almost complete. After concentration under reduced pressure, the silica gel thin-layer preparation plate was purified to obtain 65 mg of white powder with a yield of 87%. [α] D 20 =-9.5(c1.0, CHCl 3 ). 1 HNMR (400MHz, CDCl 3 )δ6.94(d,J=7.9Hz,1H),6.83–6.72(m,2H),6.67(d,J=7.1Hz,1H),6.54(d,J=7.7Hz,1H),6.50( s,1H),4.82(s,1H),4.19–4.15(m,1H),3.93–3.88(m,1H),3.85(s,3H),3.82(s,3H),3.76(s,3H) ,3.26–3.17(m,2H),3.01–2.92(m,2H),2.66–2.57(m,4H),2.47–2.39and2.39–2.45(bothm,total2H),...
Embodiment 3
[0043] Embodiment 3: synthetic compound 1-3, please refer to Figure 5 and Figure 6
[0044] .
[0045] Dissolve N-Boc-glycine (28.0 mg, 0.16 mmol) in dry toluene, add 2,4,6-trichlorobenzoyl chloride (30.5 μL, 0.20 mmol) and DIPEA (26.0 μL, 0.20 mmol), and stir for 10 min Then arctigenin (50mg, 0.134mmol) was added, DMAP (32.7mg, 0.27mmol) was added at one time, and stirring was continued at room temperature for 3h. TLC showed that the reaction was almost complete. After concentration under reduced pressure, silica gel thin-layer preparation plate was purified to obtain 59 mg of white powder with a yield of 83%. [α] D 20 =-16.3(c1.0, CHCl 3 ), 1 HNMR (400MHz, CDCl 3 ,mixtureofrotamers)δ6.95(d,J=8.0Hz,0.7H),6.81(d,J=7.9Hz,0.3H),6.76–6.72(m,1.7H),6.67–6.62(m,1H), 6.61–6.59(m,0.3H),6.54–6.51(m,1H),6.49–6.45(m,1H),5.64(br,0.3H),5.10(br,0.7H),4.21–4.15(m, 2H), 4.15–4.07(m,1H), 3.91–3.86(m,1H), 3.84(s,3H), 3.81, 3.80 and 3.74(alls, total6H), 2.99–2.88(m,2H), 2.68 –2.41...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com