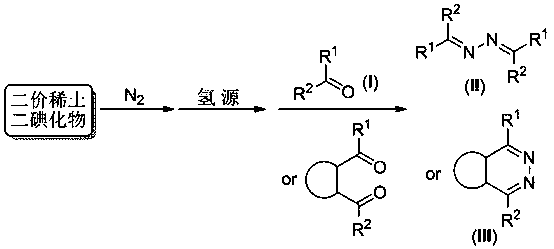
A Nitrogen Activation and Conversion Process Promoted by Divalent Rare Earth Iodides
A technology of diiodide and rare earth, applied in the fields of organic chemistry, hydrazone preparation, etc., can solve the problem that nitrogen molecules cannot be converted, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1, put dysprosium iodide (1.0 mmol) and tetrahydrofuran (15 ml) into a glass reaction bottle, react at -25 °C for 24 h under a nitrogen atmosphere of 0.1 MPa, then add 1 M HCl (3 ml), Stir for 30 minutes, then add benzaldehyde (2.0 mmol). After stirring at 70°C for 12 hours, 1M NaOH was added for neutralization. The reaction solution was extracted with ether, the solvent was removed, and the residue was purified by silica gel column chromatography to obtain 46 mg of benzaldehyde azine with a yield of 88%.
[0022] 1 H NMR (CDCl 3 , 400 MHz): 8.67(s, 2H), 7.84-7.86(m, 4H), 7.44-7.46(m, 6H). 13 C NMR (CDCl 3 , 100MHz): 162.06, 131.20, 128.79, 128.56. GC-MS: m / z=208 [M+].
Embodiment 2
[0023] Example 2, put dysprosium iodide (1.0 mmol) and 1,4-dioxane (15 ml) into a glass reaction bottle, react at -78 °C for 24 h under a nitrogen atmosphere of 0.5 MPa, and then add l M HCl (3 ml), stirred for 30 minutes, followed by the addition of acetophenone (1.0 mmol). After stirring at 70°C for 12 hours, 1M NaOH was added for neutralization. The reaction solution was extracted with ether, the solvent was removed, and the residue was purified by silica gel column chromatography to obtain 25 mg of acetophenone azine with a yield of 42%.
[0024] 1 H NMR (CDCl 3 , 400 MHz): 7.90-7.92(m, 4H), 7.42-7.44(m, 6H), 2.32(s, 6H). GC-MS: m / z=236 [M+].
Embodiment 3
[0025] Example 3, put dysprosium iodide (1.0 mmol) and diethyl ether (15 ml) into a glass reaction bottle, react at -40 °C for 24 h under a nitrogen atmosphere of 0.1 MPa, and then add 1 M H 2 SO 4 (2.5 ml), stirred for 30 minutes, followed by the addition of p-methoxybenzaldehyde (2.0 mmol). After stirring at 70°C for 12 hours, 1M NaOH was added for neutralization. The reaction solution was extracted with ether, the solvent was removed, and the residue was purified by silica gel column chromatography to obtain 41 mg of p-methoxybenzaldehyde azine with a yield of 61%.
[0026] 1 H NMR (CDCl 3 , 400 MHz): 8.61(S, 2H), 7.77(d, 4H, J = 8.67 Hz), 6.95(d,4H, J = 8.66 Hz), 3.86(s, 6H). 13 C NMR (CDCl 3 , 100MHz): 160.88, 130.12, 131.20, 55.39. GC-MS: m / z=268 [M+].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com