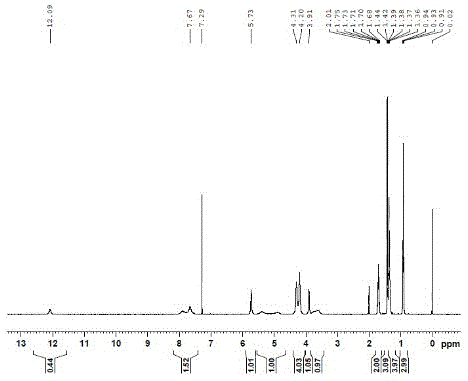
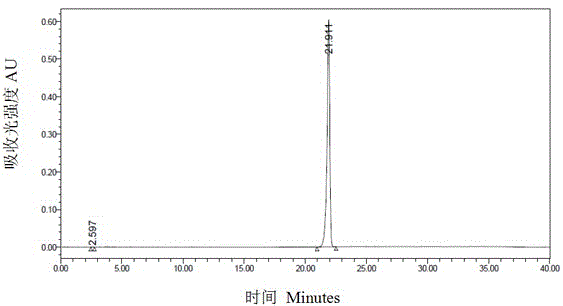
Capecitabine preparation method
The technology of capecitabine and step 2, applied in the field of medicine, can solve the problems of consumption of raw and auxiliary materials, danger to operators and the environment, and high cost, and achieve the effects of reducing environmental impact, reducing drug costs, and reducing medical expenses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
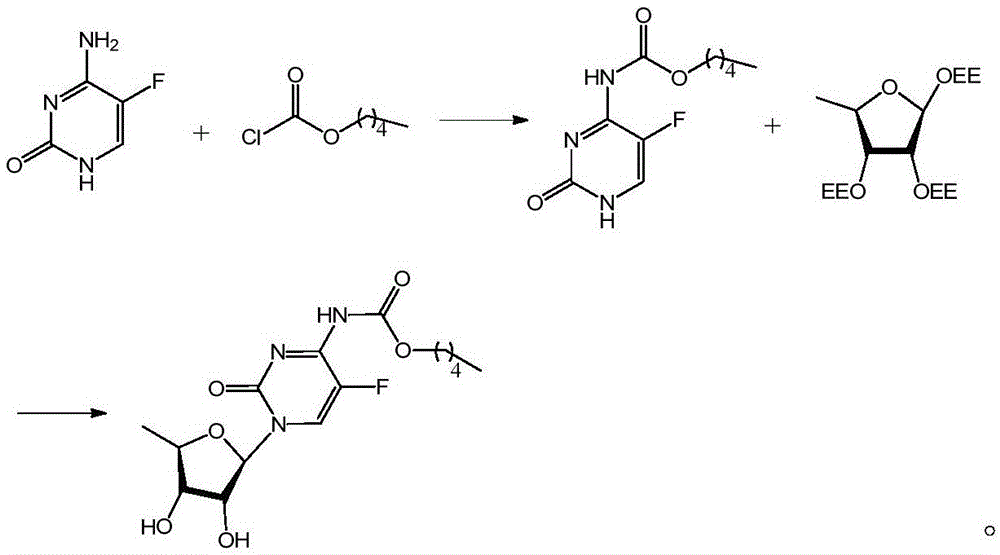
[0036] The preparation method of capecitabine of the present invention: comprise the following steps:
[0037] Step 1, using 5-FU as the initial raw material, reacting with pentoxyformyl chloride to generate an intermediate; 5-fluorocytosine: pyridine: pentoxyformyl chloride = 1:1.25~1.4:1.1~1.3, preferably 5-fluorocytosine Pyrimidine:pyridine:pentyloxycarbonyl chloride=1:1.33:1.21. The dropwise addition of pentoxycarbonyl chloride is controlled at -10°C to 0°C, preferably -10°C to -5°C. The reaction temperature is controlled at 0°C to 40°C, preferably room temperature. The concentration of hydrochloric acid used in the quenching reaction is controlled at 0.1-1.0N, preferably 0.2N. The amount of hydrochloric acid used in the quenching reaction is controlled at: HCl:5-fluorocytosine=0.25˜0.025:1, preferably HCl:5-fluorocytosine=0.05:1.
[0038] In step 2, the intermediate is coupled with a ribose derivative to generate capecitabine; the product of the first step: sugar deriv...
Embodiment 1
[0047]Add 80ml of dichloromethane into a 1L three-neck reaction flask equipped with a thermometer and a constant pressure dropping funnel, start magnetic stirring, add 3.04g of 5-fluorocytosine to the reaction flask, and then add 7.1g of pyridine. Under nitrogen protection, 11.7 g of n-amyl chloroformate was added to the constant pressure dropping funnel, and then 10 ml of dichloromethane was added. Cool the reaction solution to -10°C, start to add the dichloromethane solution of n-pentyl chloroformate dropwise, control the dropping temperature at -10°C to -5°C, remove the freezing liquid after the addition, and stir the reaction at room temperature for 2 hours. After the reaction, suck in 30ml of 0.2N hydrochloric acid, stir for 15 minutes, collect the lower dichloromethane phase, and wash the dichloromethane phase with 0.2N hydrochloric acid three times (20ml×3). Finally the dichloromethane phase was washed with water (50ml x 1). The dichloromethane phase was dried with anh...
Embodiment 2
[0050] Add 3.8 L of dichloromethane into a 10 L three-neck reaction flask equipped with a thermometer and a constant pressure dropping funnel, start mechanical stirring, add 152 g of 5-fluorocytosine, and then 122 g of pyridine into the reaction flask. Under nitrogen protection, 212 g of n-amyl chloroformate was added to the constant pressure dropping funnel, and then 200 ml of dichloromethane was added. Cool the reaction solution to -10°C, start to add the dichloromethane solution of n-pentyl chloroformate dropwise, control the dropping temperature at -10°C to -5°C, remove the freezing liquid after the addition, and stir the reaction at room temperature for 2 hours. After the reaction, suck in 1.5L of 0.2N hydrochloric acid, stir for 15 minutes, collect the lower dichloromethane phase, and wash the dichloromethane phase with 0.2N hydrochloric acid three times (500ml×3). Finally the dichloromethane phase was washed with water (500ml x 1). The dichloromethane phase was dried w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com