Construction and application of E3 region-deleted complete-copy-type recombinant adenovirus 4 carrier system
A recombinant adenovirus and vector system technology is applied in the field of construction of a complete recombinant adenovirus type 4 vector system that lacks the E3 region to replicate, and can solve the problems of reducing the application effect of the adenovirus type 5 vector, insufficient antibodies, and short-lived.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Construction of backbone plasmid pAd4FAST and preparation of electroporation competent cells
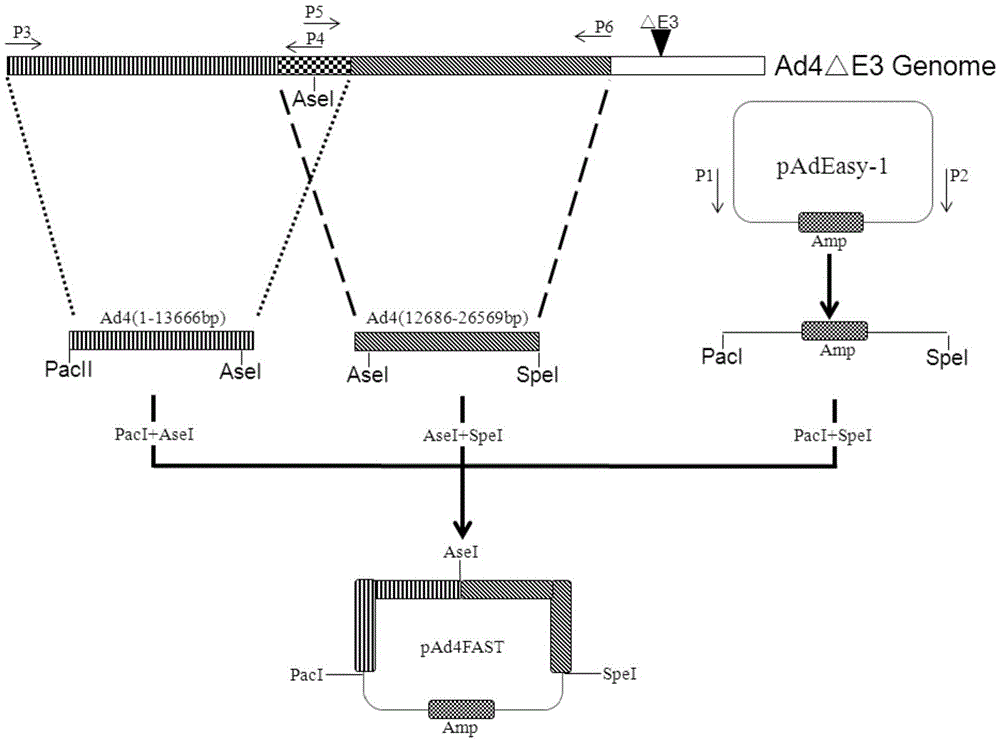
[0035] (1) Construction of backbone plasmid pAd4FAST:
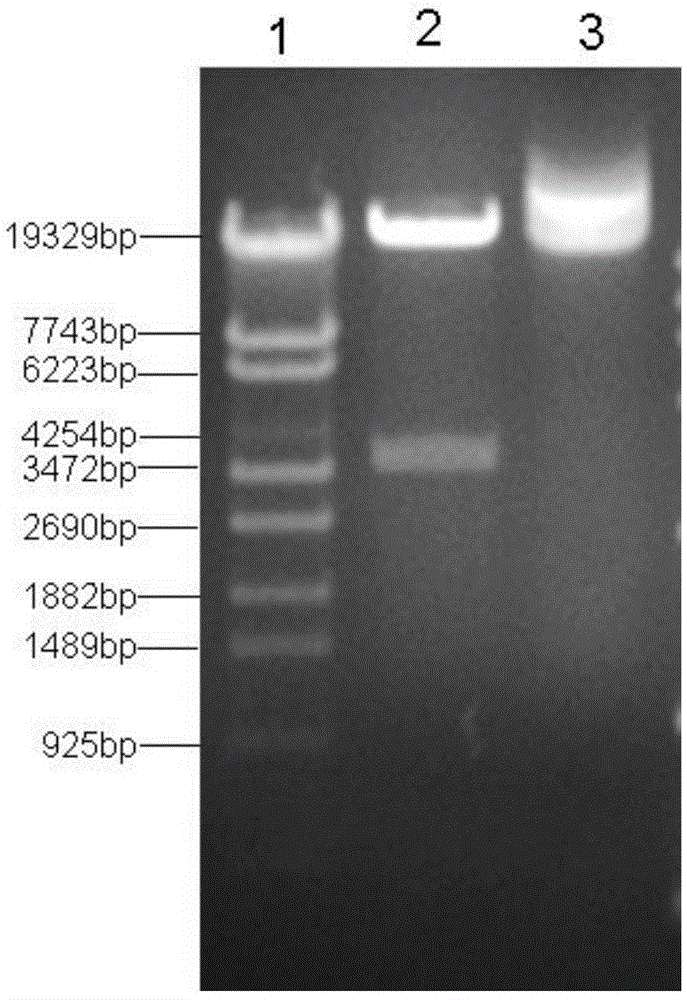
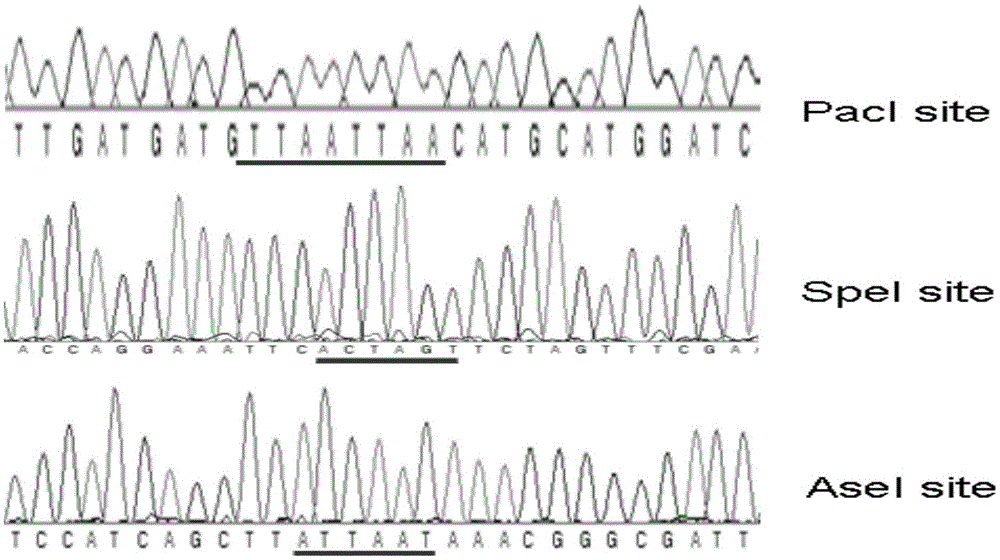
[0036] ① Using the pAdeasy-1 vector (stratagene company of the United States, GenBank accession number AY370909) as a template, use primers P1: CCTTAATTAA (PacI) CATGC (SEQ ID NO: 1) and P2: GGACTAGA (SpeI) TCTAGTTTCG (SEQ ID NO: 2) to amplify its position The fragment at 27246-31123bp (the fragment size is 3878bp), which contains the basic components of the vector such as pBR322 replication origin, ampicillin resistance gene, etc., the product is digested with PacI and SpeI, purified and recovered by DNA gel recovery kit to obtain the product 1;
[0037] Amplification was carried out in a standard polymerase chain reaction (PCR) system, the reaction system was 50 μl system: 5×PCRBuffer 10uL, template 10ng, 10mM deoxyribonucleoside triphosphate (dNTP) 1μl, upstream and downstream primers (25μM) 1μl each , high-fideli...
Embodiment 2
[0058] This example is the preparation process of a recombinant AdV4 virus carrying a gene of interest, specifically taking the preparation of "recombinant adenovirus AdV4-EGFP carrying an EGFP reporter gene" as an example:
[0059] The recombinant AdV4 adenovirus carrying the target gene can be prepared by using the vector system, and the target gene can be cloned into the cloning site of the shuttle plasmid pAd4FAST-Shuttle. After the shuttle plasmid carrying the target gene was linearized by restriction endonuclease SwaI, it was transformed into Escherichia coli BJ5183 strain (Stratagenen, USA) carrying the backbone plasmid pAd4FAST, and the target gene was obtained through homologous recombination in BJ5183 cells. The adenovirus plasmid, which is digested with the restriction endonuclease PacI, releases the recombinant adenovirus genome, and uses the method of DNA transfection to transfect the recombinant adenovirus genome into the packaging cell line HEK-293, and rescues t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com