A preparing method of albendazole
A technology of albendazole and nitroaniline, applied in the field of preparation of broad-spectrum anthelmintics, can solve problems such as poor product quality, poor environment, and large amount of sulfur-containing wastewater
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
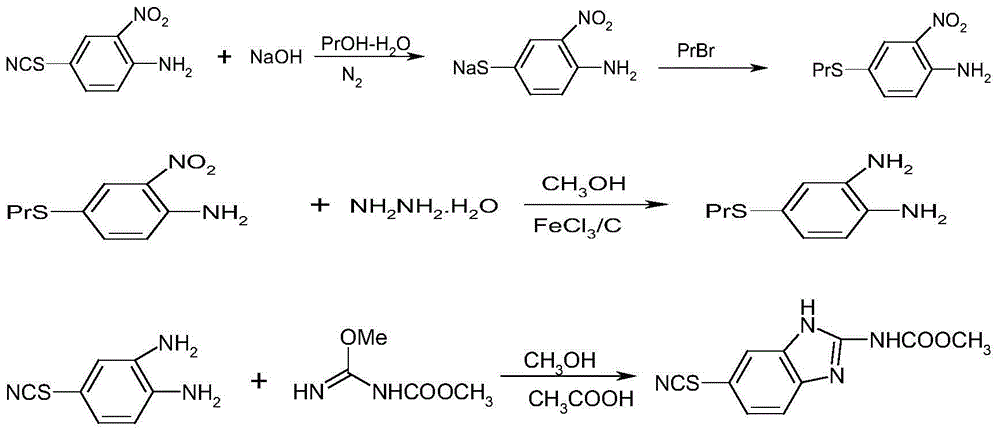
[0023] The first step, the preparation of 4-propylthio-2-nitroaniline (substitution, condensation)
[0024] Charge N 2 39.0g (0.2mol) of pure 4-thiocyanato-2-nitroaniline, 100ml of n-propanol, and 30ml of water were poured into a reaction flask under the same conditions, stirred, and 30% sodium hydroxide solution was added dropwise at 30-40°C 53.3g (0.40mol) for about 1 hour, after the dropwise addition, keep warm for 2 hours, heat up to 50°C, add 27.0g (0.22mol) bromopropane dropwise for about 0.5h, continue to keep warm for 2 hours after the dropwise addition, after the end, turn off the charger N 2 Protection, layering at 50-60°C, discarding the waste water layer, the upper layer of reddish-brown liquid is the n-propanol solution of 4-propylthio-2-nitroaniline.
[0025] The second step, the preparation (reduction) of 4-propylthio o-phenylenediamine
[0026] Put the reddish-brown liquid (4-propylthio-2-nitroaniline) in the first step into the reaction bottle, heat up, dis...
example 2
[0032] The first step, the preparation of 4-propylthio-2-nitroaniline (substitution, condensation)
[0033] Charge N 2 39.0g (0.2mol) of pure 4-thiocyanato-2-nitroaniline, 100ml of n-propanol, and 30ml of water were poured into a reaction flask under the same conditions, stirred, and 30% sodium hydroxide solution was added dropwise at 30-40°C 80g (0.60mol) for about 1 hour, after the dropwise addition, keep it warm for 2 hours, heat up to 50°C, add 37.0g (0.30mol) of bromopropane dropwise for about 0.5h, after the dropwise addition, continue to keep warm for 2 hours, after the end, turn off and fill with N 2 Protection, layering at 50-60°C, discarding the waste water layer, the upper layer of reddish-brown liquid is the n-propanol solution of 4-propylthio-2-nitroaniline.
[0034] The second step, the preparation (reduction) of 4-propylthio o-phenylenediamine
[0035] Put the reddish-brown liquid (4-propylthio-2-nitroaniline) in the first step into the reaction bottle, heat u...
example 3
[0041] The first step, the preparation of 4-propylthio-2-nitroaniline (substitution, condensation)
[0042] Charge N 2 39.0g (0.2mol) of pure 4-thiocyanato-2-nitroaniline, 100ml of n-propanol, and 30ml of water were poured into a reaction flask under the same conditions, stirred, and 30% sodium hydroxide solution was added dropwise at 30-40°C 66.7g (0.50mol) for about 1 hour, after the dropwise addition, keep it warm for 2 hours, heat up to 50°C, add 32.0g (0.26mol) of bromopropane dropwise for about 0.5h, after the dropwise addition, continue to keep warm for 2 hours, after the end, turn off the charger N 2 Protection, layering at 50-60°C, discarding the waste water layer, the upper layer of reddish-brown liquid is the n-propanol solution of 4-propylthio-2-nitroaniline.
[0043] The second step, the preparation (reduction) of 4-propylthio o-phenylenediamine
[0044]Put the reddish-brown liquid (4-propylthio-2-nitroaniline) in the first step into the reaction bottle, heat u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com