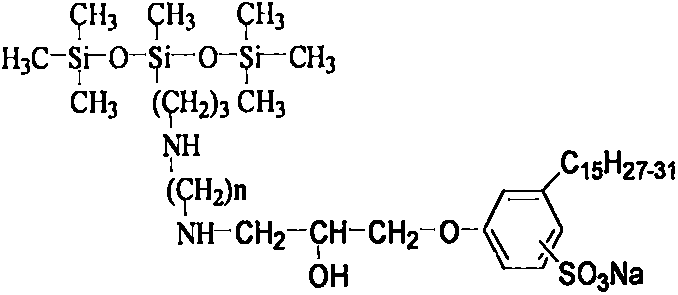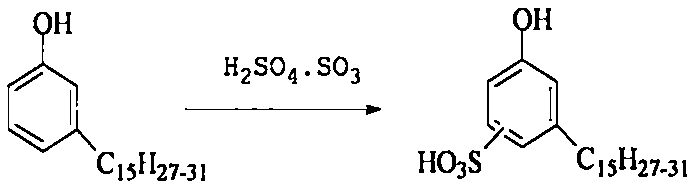Anacardol surfactant containing trisiloxane and amidogen and preparation method thereof
A surfactant, trisiloxane technology, applied in chemical instruments and methods, transportation and packaging, organic silicon compounds, etc., can solve the problems of low surface activity and few varieties, and achieve good biodegradability and human body stimulation. The effect of low resistance and ability to reduce surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
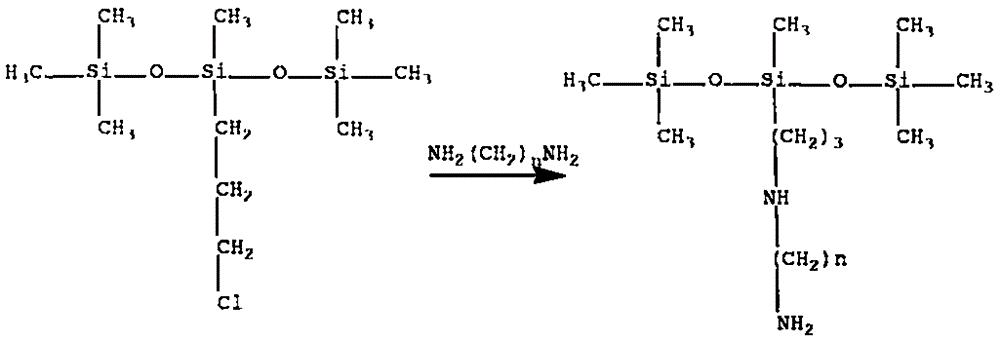
[0026] 290 g of chloropropyltrisiloxane and 74 g of propylenediamine were added to the reaction kettle, and the heating temperature was controlled at 95° C. After reacting for 7 hours, the low boilers were distilled off under reduced pressure to obtain 200 g of Intermediate 1. Sulfonate 300 g of cardanol with 190 g of fuming sulfuric acid, sulfonate at 15° C. for 3 hours, and separate the waste acid to obtain 220 g of cardanol sulfonic acid. After adding sodium hydroxide to 200g of cardanol sulfonic acid to make the pH of the solution 10, add an appropriate amount of phase transfer catalyst and dropwise add 50g of epichlorohydrin, the reaction temperature is 85°C, and the reaction time is 6 hours, and 156g of intermediate 2 is separated and obtained . Then 100 g of intermediate 1 and 85 g of intermediate 2 were reacted at 120° C. for 8 hours, separated and purified to obtain 85 g of the cardanol surfactant containing trisiloxane and amino group. The lowest surface tension was...
Embodiment 2
[0028] 250 g of chloropropyltrisiloxane and 74 g of butanediamine were added to the reaction kettle, and the heating temperature was controlled at 110° C. After reacting for 5 hours, the low boilers were distilled off under reduced pressure to obtain 220 g of intermediate 1. Sulfonate 300 g of cardanol with 195 g of fuming sulfuric acid, sulfonate at 10° C. for 3.5 hours, and separate the waste acid to obtain 245 g of cardanol sulfonic acid. After adding sodium hydroxide to 200g of cardanol sulfonic acid to make the pH of the solution 11, add an appropriate amount of phase transfer catalyst and then dropwise add 60g of epichlorohydrin, the reaction temperature is 90°C, and the reaction time is 5 hours, and 170g of intermediate 2 is separated and obtained . Then 100 g of intermediate 1 and 90 g of intermediate 2 were reacted at 115° C. for 7 hours, separated and purified to obtain 90 g of the cardanol surfactant containing trisiloxane and amino group. The lowest surface tensio...
Embodiment 3
[0030] 300 g of chloropropyltrisiloxane and 110 g of pentamethylenediamine were added to the reaction kettle, and the heating temperature was controlled at 105° C. After reacting for 5 hours, the low boilers were distilled off under reduced pressure to obtain 260 g of intermediate 1. Sulfonate 300 g of cardanol with 200 g of fuming sulfuric acid, sulfonate at 10° C. for 3.5 hours, and separate the waste acid to obtain 250 g of cardanol sulfonic acid. Add sodium hydroxide to 200g of cardanol sulfonic acid to make the pH of the solution 11, add an appropriate amount of phase transfer catalyst, and then dropwise add 65g of epichlorohydrin, the reaction temperature is 95°C, and the reaction time is 6 hours, and 156g of intermediate 2 is isolated. . Then 200 g of intermediate 1 and 125 g of intermediate 2 were reacted at 130° C. for 10 hours, separated and purified to obtain 100 g of the cardanol surfactant containing trisiloxane and amino group. The lowest surface tension was mea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com