Nonionic biomass-based surfactant and preparation method thereof
A material and quality technology, applied in the field of non-ionic biomass-based surfactants and their preparation, can solve problems such as environmental pollution and difficult degradation of surfactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
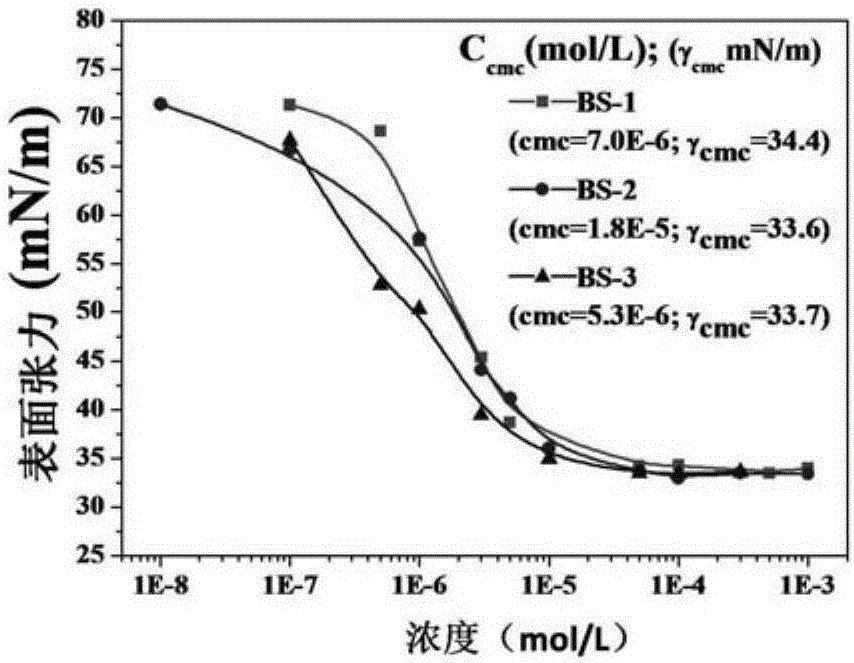
[0084] Embodiment 1, synthetic nonionic biomass-based surfactant BS-1
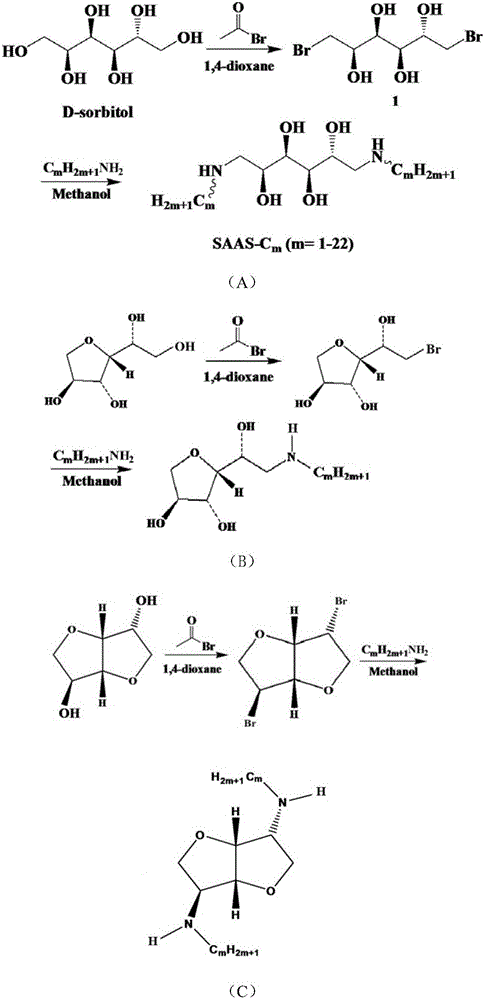
[0085] according to figure 1 (A) shown route synthetic non-ionic biomass-based surfactant, concrete steps are as follows:
[0086] (1) Add 10.1 g of D-sorbitol and 80 mL of 1,4-dioxane into the reaction vessel, stir in an ice bath, and add 10.1 mL of acetyl bromide dropwise. Adjust the reaction temperature to 18°C and stir for 14h. The solvent was distilled off under reduced pressure and extracted with dichloromethane to obtain bromosorbitol represented by formula IV-A as a yellow liquid with a yield of 85%. The structure verification data is as follows: 1 H-NMR (400MHz,D 2 O): δ=3.27(dd,1H),3.33(dd,1H),5.30(m,1H),5.41(dd,1H),5.08(m,1H),3.37(dd,1H),3.51(dd ,1H); MALDI-TOF-MS(M+H) + :Calcd.for C 6 h 12 Br 2 o 4 :307.Found:308. Verified that the structure is correct.
[0087]
[0088] (2) Add 6.2g of bromosorbitol shown in formula IV-A, 5.68g of n-octylamine, and 20mL of anhydrous methanol in...
Embodiment 2
[0091] Embodiment 2, synthetic nonionic biomass-based surfactant BS-2
[0092] according to figure 1 (A) shown route synthetic non-ionic biomass-based surfactant, concrete steps are as follows:
[0093] (1) Add 10.1 g of D-sorbitol and 80 mL of 1,4-dioxane into the reaction vessel, stir in an ice bath, and add 10.1 mL of acetyl bromide dropwise. Adjust the reaction temperature to 18°C and stir for 14h. The solvent was distilled off under reduced pressure and extracted with dichloromethane to obtain bromosorbitol represented by formula IV-A as a yellow liquid with a yield of 85%. 1 H-NMR (400MHz,D 2 O): δ=3.27(dd,1H),3.33(dd,1H),5.30(m,1H),5.41(dd,1H),5.08(m,1H),3.37(dd,1H),3.51(dd ,1H); MALDI-TOF-MS(M+H) + :Calcd.for C 6 h 12 Br 2 o 4 :307.Found:308. Verified that the structure is correct.
[0094]
[0095] (2) Add 6.2g of bromosorbitol shown in formula IV-A, 8.15g of n-dodecylamine, and 25mL of anhydrous methanol into the reaction vessel, heat up to 40°C and st...
Embodiment 3
[0097] Embodiment 3, synthetic nonionic biomass-based surfactant BS-3
[0098] according to figure 1 (C) The route shown in (C) synthesizes non-ionic biomass-based surfactant, and the specific steps are as follows:
[0099] (1) Add 10.1 g of isosorbide and 80 mL of 1,4-dioxane into a reaction vessel, stir in an ice bath, and add 10.1 mL of acetyl bromide dropwise. Adjust the reaction temperature to 18°C and stir for 14h. The solvent was distilled off under reduced pressure and extracted with dichloromethane to obtain the dehydrated bromosorbitol derivative shown in XII-A as light yellow liquid with a yield of 90%. The structure verification data is as follows: 1 H-NMR (400MHz,D 2 O): δ=3.82(dd,1H),3.88(dd,1H),5.42(m,1H),5.36(dd,1H),5.12(m,1H),3.94(dd,1H),3.86(dd ,1H); MALDI-TOF-MS: Calcd.for C 6 h 12 Br 2 o 2 :271.Found:294(M+Na); After verification, the structure is correct.
[0100]
[0101] (2) Add 8.0g of bromosorbitol dehydrated derivative shown in formula ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com