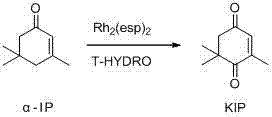
Efficient Catalytic Oxidation of α-Isophorone to Prepare Oxoisophorone
A technology for oxoisophorone and isophorone, which is applied in the field of efficient catalytic oxidation of α-isophorone to prepare oxoisophorone, can solve the problem that the catalyst cannot be recycled and reused, and achieves short reaction time. , The effect of high reaction selectivity and reduction of energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 0.54g (4mmol) α-isophorone, 3mg (0.004mmol) Rh 2 (esp) 2 , 2.8ml (20mmol) 70% tert-butyl hydroperoxide aqueous solution was added to a 10ml round bottom flask and stirred at room temperature for 24h. The measured conversion was 91%, and the isolated yield was 78%.
Embodiment 2-3
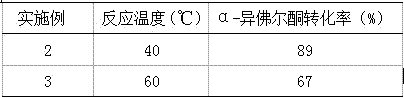
[0023] Similar to Example 1, the reaction temperature was changed, and the reaction was carried out at room temperature for 24 hours. The results are shown in Table 1.
[0024] Table 1 Effects of different reaction temperatures on the oxidation reaction of α-isophorone.
[0025]
Embodiment 4-9
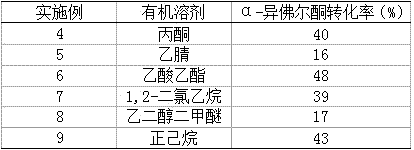
[0027] Similar to Example 1, 2 ml of different organic solvents were added, tert-butyl hydroperoxide decane solution was used as an oxidizing agent, and the reaction was carried out at room temperature for 24 hours. The results are shown in Table 2.
[0028] Table 2 Effect of different organic solvents on the oxidation of α-isophorone.
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com