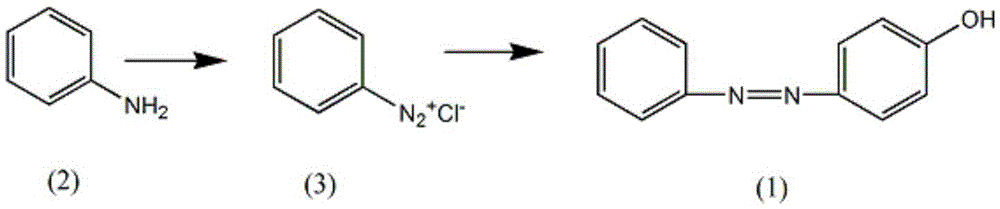
Synthetic method for oxyphenbutazone drug intermediate-4-hydroxyazobenzene
A technology of hydroxyazobenzene and hydroxybutazone, applied in organic chemistry and other directions, can solve problems such as weak antipyretic and analgesic effects, and achieve the effects of reducing reaction temperature and reaction time, reducing intermediate links and improving reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0013] (i) Add 300ml of sulfuric acid solution with a mass fraction of 5% in the reaction vessel equipped with agitator, thermometer and dropping funnel, control the stirring speed at 100rpm, slowly add 1.2mol of aniline, and reduce the solution temperature to 6°C after completely dissolving, Add dropwise 1.1 mol of potassium bisulfite dissolved in 200 ml of water to form a solution. During the dissolution process, the temperature of the solution is controlled at 6° C., and the end point of the reaction is measured with potassium iodide test paper to generate diazonium salt (3);
[0014] (ii) Add 2L of potassium carbonate solution with a mass fraction of 6% and 1.2mol of phenol in another container to form a potassium phenate solution, slowly add the diazotization solution obtained in step (i) at a speed of 100 rpm, Control the temperature of the solution at 16°C, continue to react for 30 minutes after adding, add the adjusting solution to keep the pH of the solution at 9, then...
example 2
[0016] (i) Add 300ml of sulfuric acid solution with a mass fraction of 7% in the reaction vessel equipped with stirrer, thermometer and dropping funnel, control the stirring speed at 150rpm, slowly add 1.2mol of aniline, and reduce the solution temperature to 7°C after completely dissolving, Add dropwise 1.1 mol of potassium bisulfite dissolved in 220 ml of water to form a solution. During the dissolution process, the temperature of the solution is controlled at 7° C., and the end point of the reaction is measured with potassium iodide test paper to generate diazonium salt (3);
[0017] (ii) Add 2.1 L of potassium carbonate solution with a mass fraction of 8% and 1.2 mol of phenol in another container to form a potassium phenate solution, and slowly add the diazotization solution obtained in step (i) at a stirring speed of 130 rpm , control the temperature of the solution at 18°C, continue to react for 35 minutes after adding, add the adjusting solution to keep the pH of the so...
example 3
[0019] (i) Add 300ml of sulfuric acid solution with a mass fraction of 10% in the reaction vessel equipped with stirrer, thermometer and dropping funnel, control the stirring speed at 200rpm, slowly add 1.2mol of aniline, and reduce the solution temperature to 9°C after completely dissolving, Add dropwise 1.1 mol of potassium bisulfite dissolved in 230 ml of water to form a solution. During the dissolution process, the temperature of the solution is controlled at 8° C., and the end point of the reaction is measured with potassium iodide test paper to generate diazonium salt (3);
[0020] (ii) Add 2.3 L of potassium carbonate solution with a mass fraction of 9% and 1.2 mol of phenol in another container to form a potassium phenate solution, slowly add the diazotization solution obtained in step (i) at a stirring speed of 160 rpm, Control the temperature of the solution at 20°C, continue to react for 40 minutes after adding, add the adjusting solution to keep the pH of the soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com