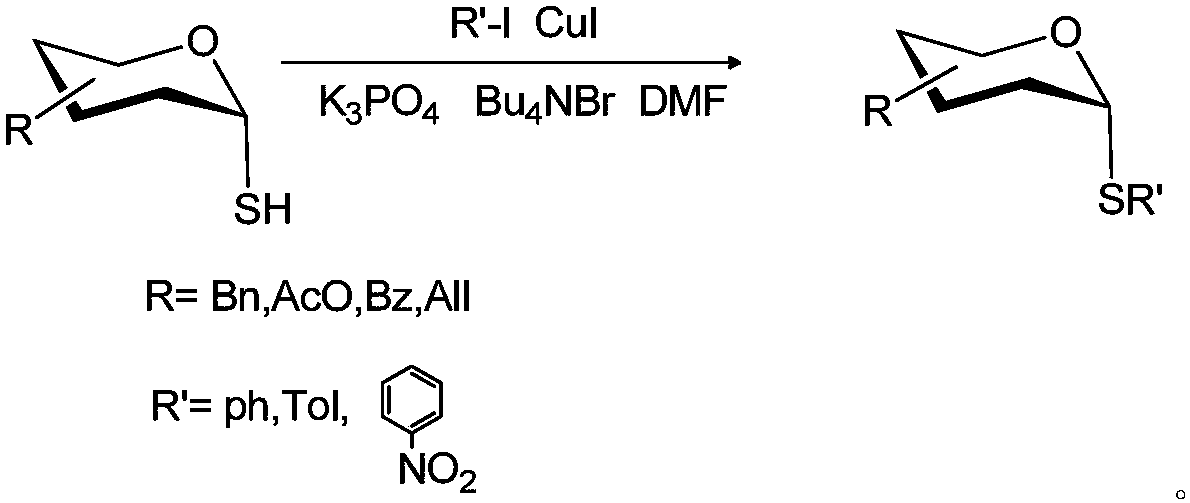
A method for synthesizing α-aryl glucosinolates
A technology of aryl glucosinolates and glycosyl thiols, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve problems such as large-scale synthesis difficulties and high reactivity of β-halogenated sugars, and achieve Overcome the effects of expensive reagents, easy separation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
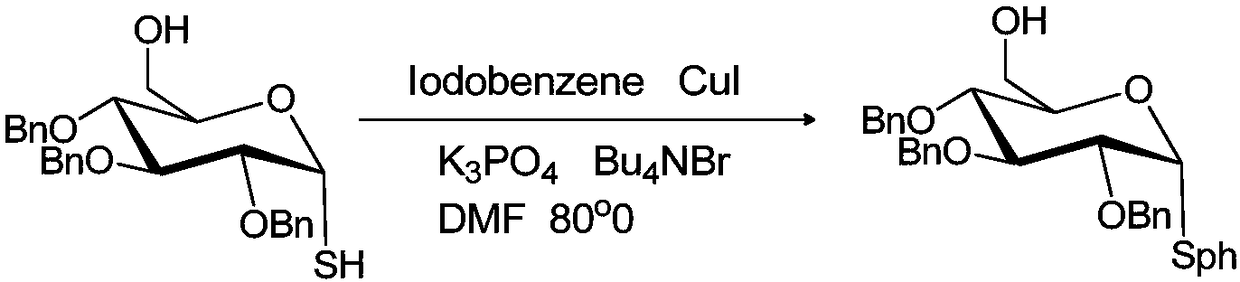
[0015] Example 1: Preparation of 6-O-hydroxyl-2,3,4-tri-O-benzyl-1-S-phenyl-α-D-glucose
[0016] The reaction formula is:
[0017]
[0018] At room temperature, add 16mg (10mol%) cuprous iodide, 177mg (0.8mmol) potassium phosphate, 42mg (0.13mmol) tetrabutylammonium bromide to a 50ml two-neck round bottom flask, vacuumize, and fill with nitrogen Three times, and then inject 0.47g (1mmol) α-glycosylthiol glucose and 0.17g (0.8mmol) iodobenzene solution dissolved in N,N dimethylformamide solvent into the circle with a disposable syringe under nitrogen protection. in the bottom flask. The round bottom flask was placed in an oil bath, and the temperature was raised to 80°C for 4 hours, and the reaction process was tracked by thin layer chromatography. After the reaction, cool down to room temperature, add saturated brine to the round bottom flask to quench the reaction, add an appropriate amount of saturated brine and ethyl acetate, extract and separate the liquids, combine t...
Embodiment 2
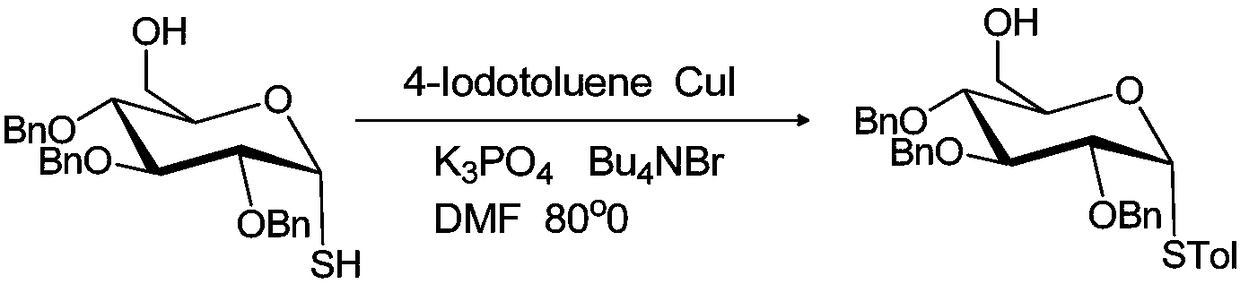
[0019] Example 2: Preparation of 6-O-hydroxyl-2,3,4-tri-O-benzyl-1-S-p-methylphenyl-α-D-glucose
[0020] The reaction formula is:
[0021]
[0022] At room temperature, add 16mg (10mol%) cuprous iodide, 177mg (0.8mmol) potassium phosphate, 42mg (0.13mmol) tetrabutylammonium bromide, 183mg (0.8mmol) in the two-neck round bottom flask of 50 milliliters 4-iodotoluene, evacuated, filled with nitrogen three times, and then injected 0.47g (1mmol) α-glycosylthiol glucose solution dissolved in N,N dimethylformamide solvent into in a round bottom flask. The round bottom flask was placed in an oil bath, and the temperature was raised to 80°C for 4 hours, and the reaction process was tracked by thin layer chromatography. After the reaction, cool down to room temperature, add saturated brine to the round bottom flask to quench the reaction, add an appropriate amount of saturated brine and ethyl acetate, extract and separate the liquids, combine the organic phases, and then dry with a...
Embodiment 3
[0023] Example 3: Preparation of 6-O-hydroxyl-2,3,4-tri-O-benzyl-1-S-p-nitrophenyl-α-D-glucose
[0024] The reaction formula is:
[0025]
[0026] At room temperature, add 16mg (10mol%) cuprous iodide, 1177mg (0.8mmol) potassium phosphate, 42mg (0.13mmol) tetrabutylammonium bromide, 209mg (0.8mmol) in the two-neck round bottom flask of 50 milliliters 4-iodotoluene, evacuated, filled with nitrogen three times, and then injected 0.47g (1 mmol) of α-glycosylthiol glucose solution dissolved in N,N dimethylformamide solvent into in a round bottom flask. The round bottom flask was placed in an oil bath, and the temperature was raised to 80°C for 4 hours, and the reaction process was tracked by thin layer chromatography. After the reaction, cool down to room temperature, add saturated brine to the round bottom flask to quench the reaction, add appropriate amount of saturated brine and ethyl acetate, extract and separate the liquids, combine the organic phases, and then dry with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com