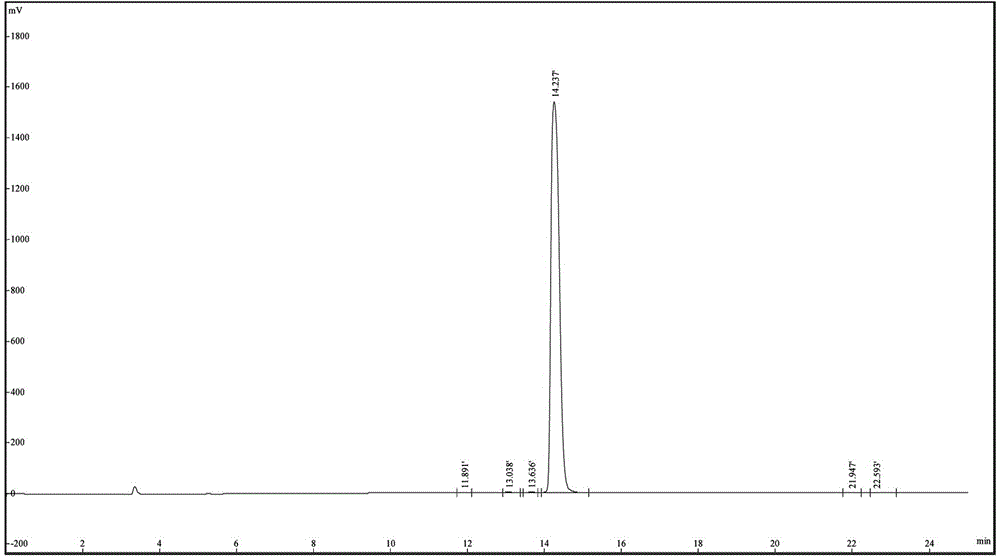
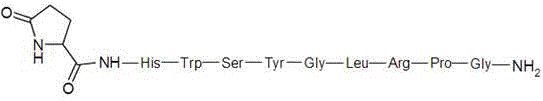Gonadorelin purification method
A volume percentage and gradient technology, applied in the field of gonadorelin purification, can solve the problems of low purity of crude peptide, low yield, difficult product control, etc., and achieve the effect of reducing cost, increasing sample loading, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. Sample Processing
[0019] Weigh 5 g of crude gonadorelin and add 5% acetonitrile by volume to dissolve it ultrasonically until the liquid is clear, filter through a filter membrane with a pore size of 0.45 um, and collect the filtrate for later use.
[0020] 2. Primary purification
[0021] Chromatographic column: SPE column. Purification process: put the above filtrate on the SPE column, the SPE column is HyperSepPEP, first eluted with 10% acetonitrile + 0.1% TFA aqueous solution by volume, and then eluted with 40% acetonitrile + 0.1% TFA aqueous solution by volume, Collect the peptide solution of the peak of interest. Concentrate the collected fractions to 40-50 mg / mL by rotary evaporation under reduced pressure.
[0022] 3. Secondary purification
[0023] Chromatographic column: C18 chromatographic column, column diameter and length: 50cm×25cm. Mobile phase: mobile phase A is 0.01-0.5% by volume phosphoric acid aqueous solution, mobile phase B is chromatograp...
Embodiment 2
[0027] 1. Sample Processing
[0028] Weigh 15g of crude gonadorelin and add 5% acetonitrile by volume to dissolve it ultrasonically until the liquid is clear, filter through a filter membrane with a pore size of 0.45um, and collect the filtrate for later use.
[0029] 2. Primary purification
[0030] Chromatographic column: SPE column. Purification process: put the above filtrate on the SPE column, which is an MCXSPE column, first elute with 10% acetonitrile + 0.1% TFA aqueous solution by volume, then elute with 40% acetonitrile + 0.1% TFA aqueous solution by volume, and collect Peptide solution of the target peak. Concentrate the collected fractions to 40-50 mg / mL by rotary evaporation under reduced pressure.
[0031] 3. Secondary purification
[0032] Chromatographic column: C18 chromatographic column, column diameter and length: 100cm×25cm. Mobile phase: mobile phase A is 0.01-0.5% by volume phosphoric acid aqueous solution, mobile phase B is chromatographically pure ac...
Embodiment 3
[0036] 1. Sample Processing
[0037] Weigh 25g of crude gonadorelin and add 5% acetonitrile by volume to dissolve it ultrasonically until the liquid is clear, filter through a filter membrane with a pore size of 0.45um, and collect the filtrate for later use.
[0038] 2. Primary purification
[0039] Chromatographic column: SPE column. Purification process: put the above filtrate on the SPE column, the SPE column is HyperSepPEP, first eluted with 10% acetonitrile + 0.1% TFA aqueous solution by volume, and then eluted with 40% acetonitrile + 0.1% TFA aqueous solution by volume, Collect the peptide solution of the peak of interest. Concentrate the collected fractions to 40-50 mg / mL by rotary evaporation under reduced pressure.
[0040] 3. Secondary purification
[0041]Chromatographic column: C18 chromatographic column, column diameter and length: 150cm×25cm. Mobile phase: mobile phase A is 0.01-0.5% by volume phosphoric acid aqueous solution, mobile phase B is chromatograph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com