New electroluminescent material and application thereof
An electroluminescent material, a new type of technology, applied in the direction of luminescent materials, circuits, electrical components, etc., to achieve good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
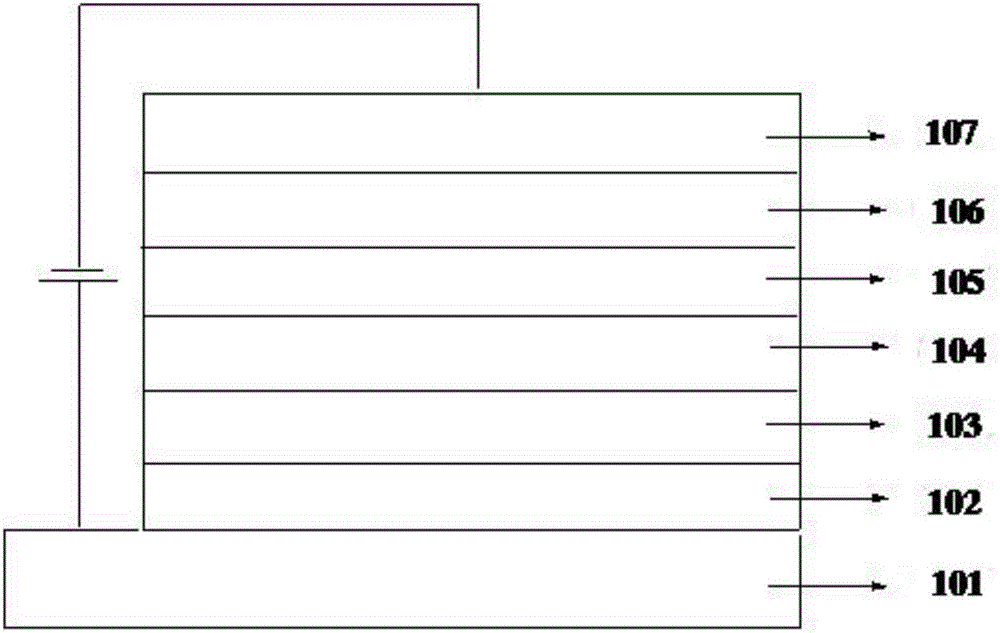
Image
Examples
Embodiment 1
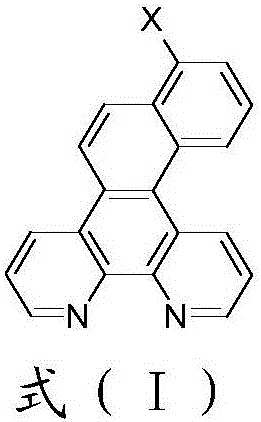
[0040] The preparation of embodiment 1 intermediate 7-bromonaphthophenanthroline (M5)
[0041]
[0042] In a 500mL three-necked flask, add 5-bromophenanthroline (10.24g, 0.04mol), pinacol bisboronic acid ester (11.18g, 0.044mol), potassium carbonate (16.60g, 0.12mol), toluene (100g ), industrial methanol (100g), H 2 O (150g), stirred under nitrogen protection for 30min, added Pd(PPh 3 ) 4 (0.46g, 0.4mmol), reflux for 8h. After cooling down to room temperature, the aqueous phase was separated, the organic phase was washed once with 200 g of water, the organic phase was passed through a silica gel column to remove residual catalyst and mechanical impurities, and the column liquid was decompressed to remove the solvent to obtain 10.52 g of intermediate M1 with a crude yield of 86%. Next reaction.
[0043] In a 500mL three-necked flask, add o-bromoiodobenzene (11.28g, 0.04mol), trimethylsilylacetylene (3.92g, 0.04mol), triethylamine (200g), Pd(PPh 3 ) 4 Cl 2 (0.28g, 0.4m...
Embodiment 2
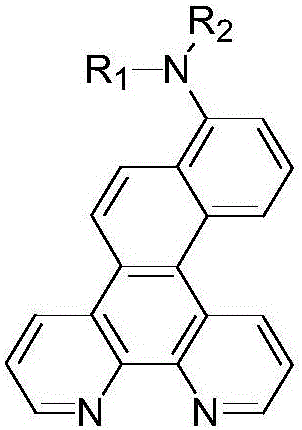
[0047] The preparation of embodiment 2 compound 1
[0048]
[0049] Add intermediate M5 (1.8g, 5mmol) prepared in Example 1, phenylboronic acid (0.67g, 5.5mmol), potassium carbonate (1.38g, 10mmol), tetrakis (triphenylphosphine) palladium (0.28 g) in the 100ml there-necked flask g, 0.25mmol), toluene (15mL), absolute ethanol (15mL), H 2 O (10mL), reflux reaction under nitrogen protection for 8h. After cooling down to room temperature, the reaction solution was spin-dried to obtain 1.28 g of white solid with a yield of 72% by column chromatography. MS (m / z): [M + ]=355.21, molecular formula C 26 h 16 N 2 , the theoretical value is 356.13.
Embodiment 3
[0050] The preparation of embodiment 3 compound 3
[0051]
[0052] In the 100ml there-necked flask, add the intermediate M5 (1.8g, 5mmol) prepared in Example 1, 4-phenyl 1-naphthalene boronic acid (1.36g, 5.5mmol), potassium carbonate (1.38g, 10mmol), tetrakis(triphenyl Phosphine) palladium (0.28g, 0.25mmol), toluene (15mL), absolute ethanol (15mL), H 2 O (10mL), reflux reaction under nitrogen protection for 8h. After cooling down to room temperature, the reaction solution was spin-dried to obtain 1.22 g of a white solid with a yield of 51% by column chromatography. MS (m / z): [M + ]=481.29, molecular formula C 36 h 22 N 2 , the theoretical value is 482.18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com