Aripiprazole long-acting suspension and preparation method thereof
A technology of aripiprazole and suspension, which is applied in the field of medicine, can solve the problems of poor dispersibility of aripiprazole freeze-dried suspension, and achieve the effects of improving drug safety, improving preparation stability, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
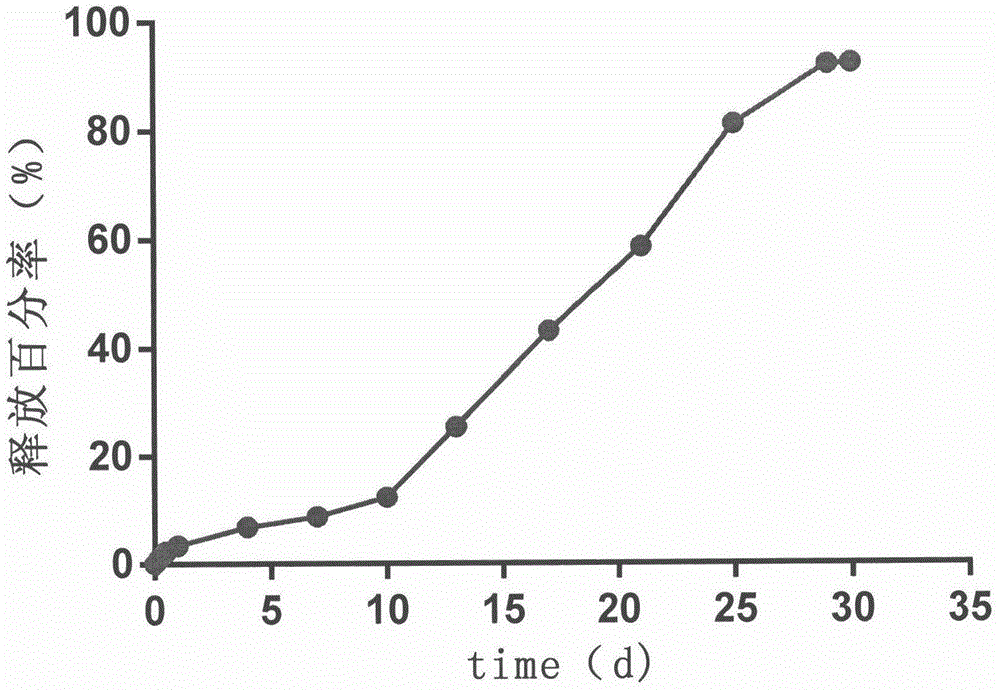
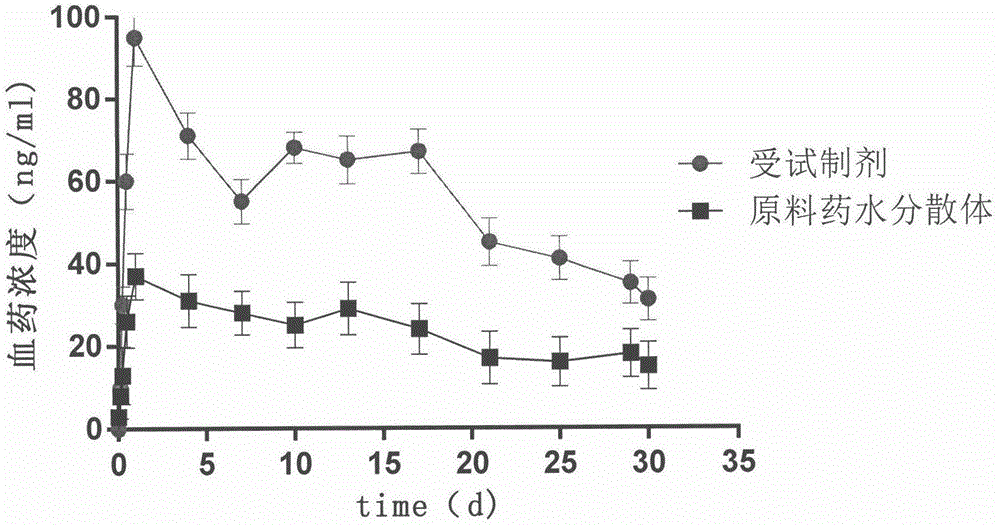
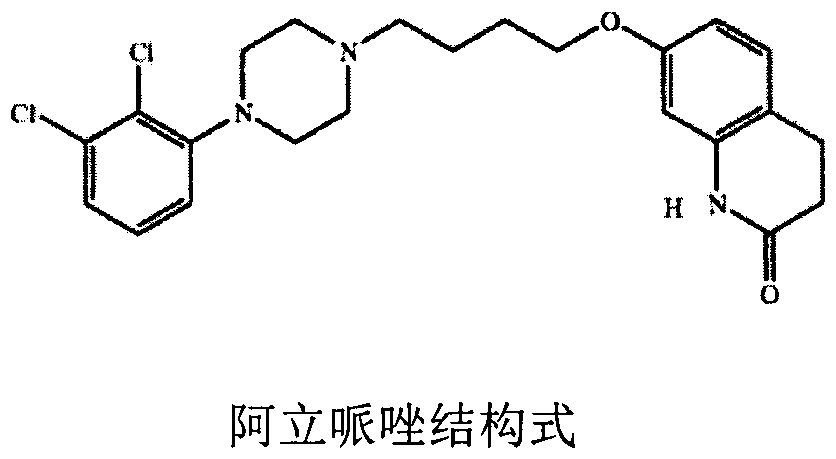
Image
Examples
Embodiment 1
[0038] Embodiment 1: Preparation of Aripiprazole Long-acting Suspension (400mg / 2ml)
[0039]
[0040] First, the aripiprazole was pulverized with a jet mill (MX-50 supersonic jet mill, Yixing Juneng Superfine Pulverization Equipment Co., Ltd.), with an average particle size of 1-15 μm. Then, add 100 g of pulverized aripiprazole, 201.25 g of polysorbate and appropriate amount of water into a horizontal grinder (SWZX-0.4 horizontal grinder, Shanghai Shihe Electromechanical Equipment Co., Ltd.), and start grinding at 20 Hz. Grind for 10 minutes to pre-disperse the drug with water, add 3.125 g of sodium carboxymethylcellulose, 18.75 g of mannitol, 0.2 g of sodium dihydrogen phosphate monohydrate and the remaining water, increase the grinding speed, and continue grinding for 30 minutes at 45 Hz. Adjust the pH to 7 with 2N sodium hydroxide solution to obtain a preliminary suspension. Finally, the sample was transferred to a three-dimensional movable colloid mill, and circulated ...
Embodiment 2
[0043] Embodiment 2: Preparation of Aripiprazole Long-acting Suspension (300mg / 2ml)
[0044]
[0045] First, the aripiprazole was pulverized with a jet mill (MX-50 supersonic jet mill, Yixing Juneng Superfine Pulverization Equipment Co., Ltd.), with an average particle size of 1-15 μm. Then, 75g of crushed aripiprazole, 201.25g of polysorbate and appropriate amount of water were added to a horizontal grinder (SWZX-0.4 horizontal grinder, Shanghai Siehe Electromechanical Equipment Co., Ltd.), and the grinding was started at 20 Hz. Grind for 10 minutes to pre-disperse the drug with water, add 3.125 g of sodium carboxymethylcellulose, 18.75 g of mannitol, 0.2 g of sodium dihydrogen phosphate monohydrate and the remaining water, increase the grinding speed, and continue grinding for 30 minutes at 45 Hz. Adjust the pH to 7 with 2N sodium hydroxide solution to obtain a preliminary suspension. Finally, the sample was transferred to a three-dimensional movable colloid mill, and ci...
Embodiment 3
[0048] Embodiment 3: Preparation of Aripiprazole Long-acting Suspension (400mg / 2ml)
[0049]
[0050]
[0051] First, the aripiprazole was pulverized with a jet mill (MX-50 supersonic jet mill, Yixing Juneng Superfine Pulverization Equipment Co., Ltd.), with an average particle size of 1-15 μm. Then, add 100 g of pulverized aripiprazole, 201.25 g of polysorbate and appropriate amount of water into a horizontal grinder (SWZX-0.4 horizontal grinder, Shanghai Shihe Electromechanical Equipment Co., Ltd.), and start grinding at 20 Hz. Grind for 10 minutes to pre-disperse the drug with water, add 6.25 g of sodium carboxymethylcellulose, 18.75 g of mannitol, 0.2 g of sodium dihydrogen phosphate monohydrate and the remaining water, increase the grinding speed, and continue grinding for 30 minutes at 45 Hz. Adjust the pH to 7 with 2N sodium hydroxide solution to obtain a preliminary suspension. Finally, the sample was transferred to a three-dimensional movable colloid mill, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com