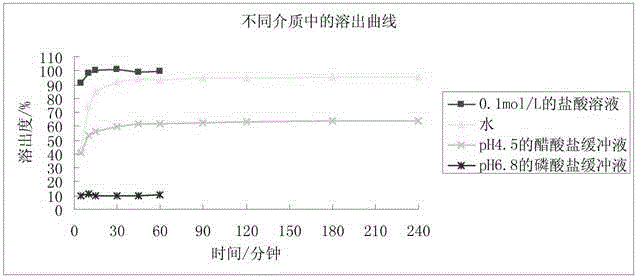
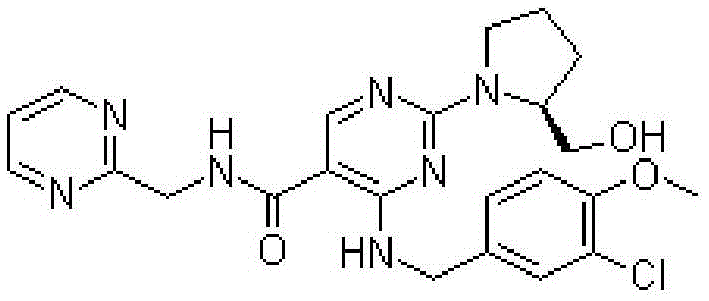
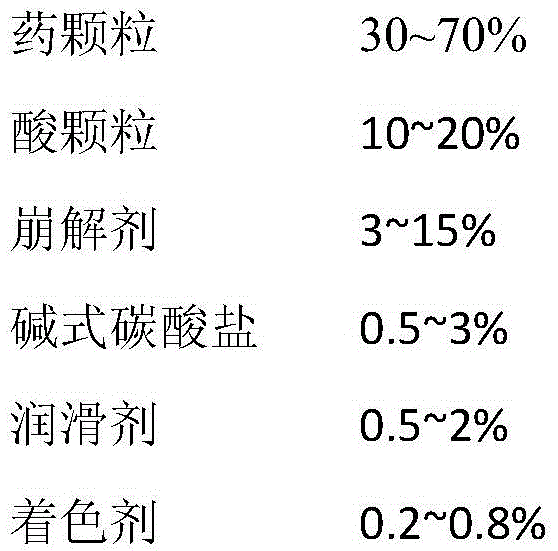
Avanafil tablet and preparation method thereof
A kind of avanafil, non-tablet technology, applied in the field of pharmaceutical preparations, can solve the problems of low dissolution rate, affecting drug bioavailability and curative effect, and slow dissolution, so as to optimize the uneven content and improve the slow dissolution of drugs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] This embodiment relates to a kind of avanafil tablet and preparation method thereof:
[0051] Composition of prescription components:
[0052]
[0053]
[0054] Preparation:
[0055] 1. get above-mentioned consumption avanafil crude drug and mannitol and mix 80 mesh sieves, then adopt fluidized bed top spray technology, add binding agent 10% (w / w) hydroxypropyl cellulose aqueous solution, granulate, Pass through a 20-mesh sieve for granulation to obtain drug granules;
[0056] II. Take the above-mentioned amount of fumaric acid, then adopt a fluidized bed top spraying process, add a binder 10% (w / w) hydroxypropyl cellulose aqueous solution, granulate, pass through a 20-mesh sieve for granulation, and obtain acid granules;
[0057] III. The drug granules and acid granules obtained in I and II are added with the above-mentioned amount of low-substituted hydroxypropyl cellulose, light calcium carbonate, magnesium stearate, and yellow iron oxide, mixed, and compress...
Embodiment 2
[0059] This embodiment relates to a kind of avanafil tablet and preparation method thereof:
[0060] Composition of prescription components:
[0061]
[0062]
[0063] Preparation:
[0064] 1. get above-mentioned consumption avanafil crude drug and mannitol and mix 80 mesh sieves, then adopt fluidized bed top spray technology, add binding agent 10% (w / w) hydroxypropyl cellulose aqueous solution, granulate, Pass through a 20-mesh sieve for granulation to obtain drug granules;
[0065] II. Take the above-mentioned amount of fumaric acid, then adopt a fluidized bed top spraying process, add a binder 10% (w / w) hydroxypropyl cellulose aqueous solution, granulate, pass through a 20-mesh sieve for granulation, and obtain acid granules;
[0066] III. The drug granules and acid granules obtained in I and II are added with the above-mentioned amount of low-substituted hydroxypropyl cellulose, light calcium carbonate, magnesium stearate, and yellow iron oxide, mixed, and compress...
Embodiment 3
[0068] This embodiment relates to a kind of avanafil tablet and preparation method thereof:
[0069] Composition of prescription components:
[0070]
[0071] Preparation:
[0072] 1. get above-mentioned consumption avanafil crude drug and mannitol and mix 80 mesh sieves, then adopt fluidized bed top spray technology, add binding agent 10% (w / w) hydroxypropyl cellulose aqueous solution, granulate, Pass through a 20-mesh sieve for granulation to obtain drug granules;
[0073]II. Take the above-mentioned amount of fumaric acid, then adopt a fluidized bed top spraying process, add a binder 10% (w / w) hydroxypropyl cellulose aqueous solution, granulate, pass through a 20-mesh sieve for granulation, and obtain acid granules;
[0074] III. The drug granules and acid granules obtained in I and II are added with the above-mentioned amount of low-substituted hydroxypropyl cellulose, light calcium carbonate, magnesium stearate, and yellow iron oxide, mixed, and compressed into table...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com