A minimally invasive intervention method for establishing a heart failure animal model
A minimally invasive intervention and animal model technology, applied in the field of biomedicine, can solve problems such as the difficulty of suturing and fixing pacemaker electrodes, the animal survival rate of less than 60%, and the difficulty of thoracotomy surgery, so as to facilitate physical recovery , easy anti-infection treatment, beneficial to healing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Minimally invasive intervention method for installing canine pacemaker
[0037] 1. Anesthesia
[0038] Inject 3% sodium pentobarbital solution intravenously, 1mL / kg, that is, 30mg / kg, observe the breathing of the experimental dogs.
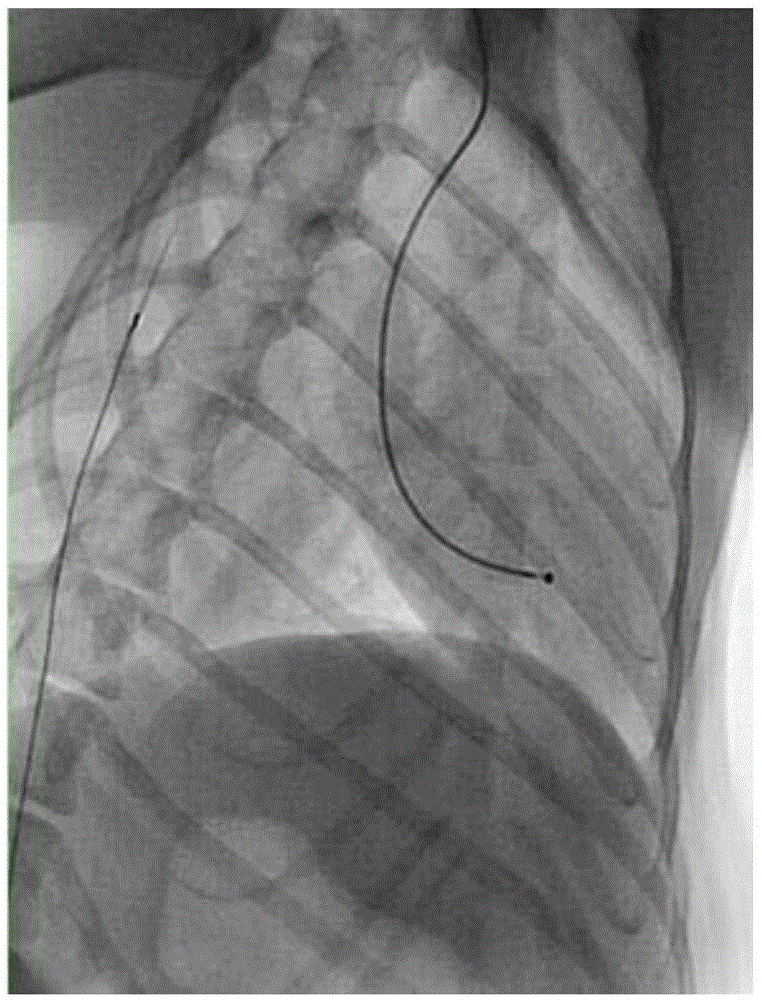
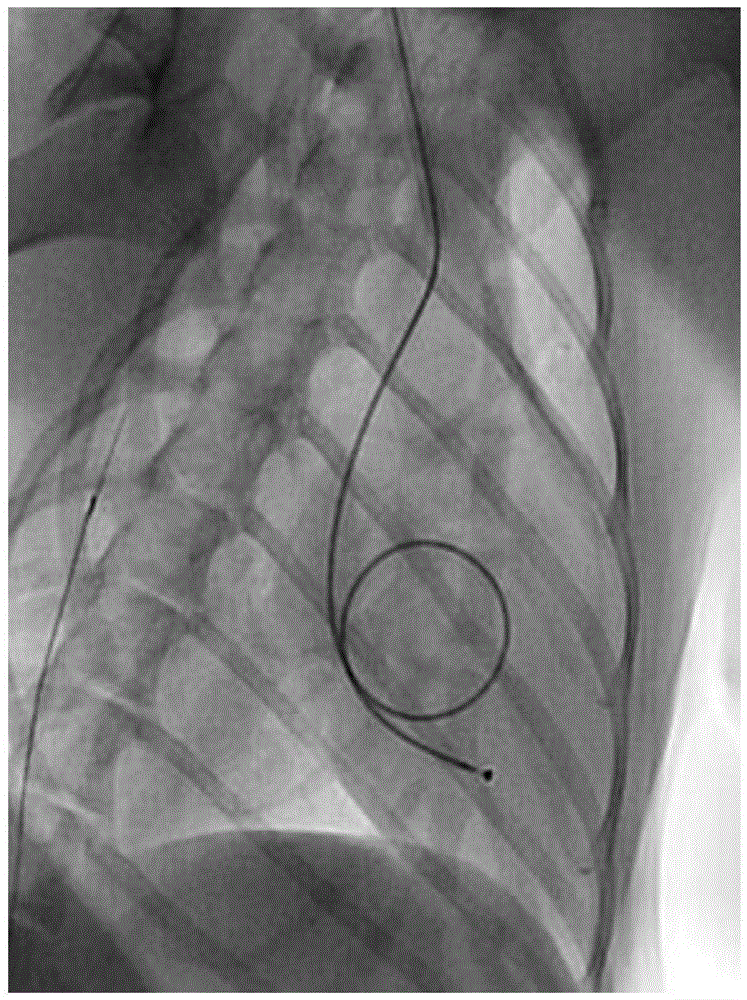
[0039] 2. Install pacemaker electrodes
[0040] The dog was placed in a lying position on the left side, and the right neck was shaved, 75% alcohol was used for skin preparation, and iodophor disinfection. Use a scalpel to make an incision parallel to the skin about 2 cm parallel to the body, and use gauze to stop bleeding. The right common jugular vein was bluntly separated, the common jugular vein was punctured with a vascular puncture needle, and a 5F catheter sheath was inserted to establish a vascular cannula. The distal end of the right common jugular vein was completely ligated with sutures, and the blood vessel wall of the proximal right common jugular vein was slightly tightened and fixed with the catheter sheath with sutures....
Embodiment 2
[0045] Example 2: Establishment of a model of canine heart failure
[0046] 1. Color Doppler Doppler Inspection Results
[0047] Pacing time
End diastolic volume EDV (mL)
End-systolic volume ESV (mL)
Stroke volume per minute SV (mL)
Left ventricular fractional shortening FS (%)
Ejection fraction EF (%)
Before pacing
19
6
14
38
71
Pacing for 1 week
45
16
29
34
64
Pacing for 2 weeks
84
46
38
22
46
Pacing for 3 weeks
56
33
23
20
41
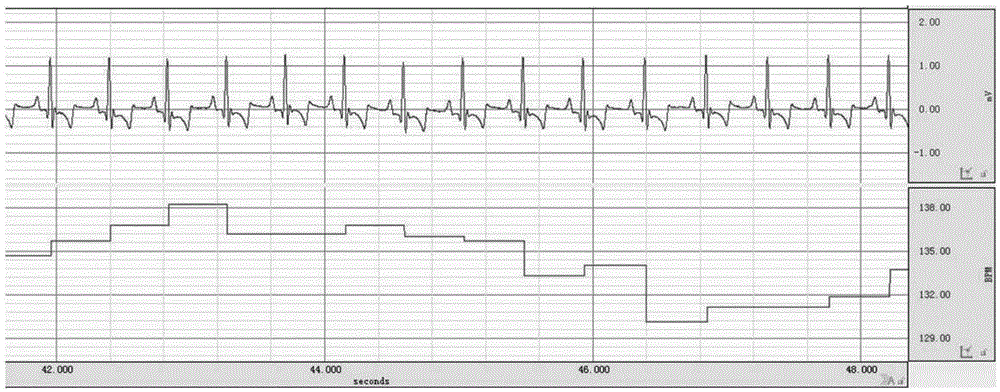
[0048] 2. ECG examination results of canine heart failure model
[0049] Install a pacemaker to establish a dog heart failure model. ECG before pacing ( image 3 ), ECG after 1 week of pacing ( Figure 4 ), ECG after 2 weeks of pacing ( Figure 5 ), ECG after 3 weeks of pacing ( Image 6 ).
[0050] 3. Myocardial enzyme test results of canine heart failure model
[0051] Pacing time
ALT (U / L)
AST (U / L)
CK (U / L)
CKMB (U / L)
LDH (U / L)
UERA (mmol / L)
CREA (μmol / L)
TBIL (μmol / L)
Before pacing
27.75
34.85
358.80
349.70
106.60
2.92
37...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com