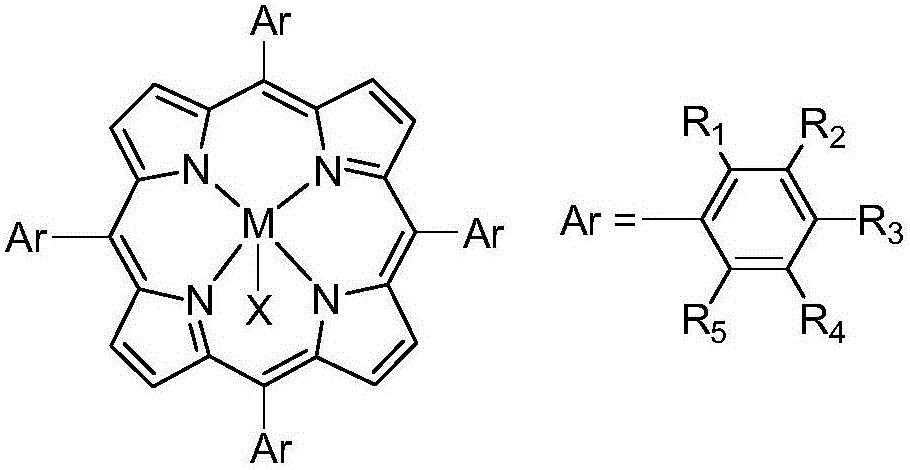
Catalyst for catalyzing alkene epoxidation
A catalyst and epoxidation technology, applied in physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, catalytic reactions, etc., can solve the problems of high cost and difficulty in recycling catalysts, and achieve easy reuse , superior economy, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1g of hydroxylated carbon nanotubes was added to anhydrous DMF, and the reaction solution was ultrasonicated for 6h. After the reactants were uniformly dispersed, the metalloporphyrin (M=Fe, R 2 = NO 2 , R 1 =R 3 =R 4 =R 5 =H, X=Cl) 0.1 g was added to the reaction solution, heated by an oil bath to reflux, after 8 hours, the reaction solution was cooled to room temperature, filtered through a polytetrafluoroethylene microporous membrane, and washed three times with tetrahydrofuran until no After repeating the operation many times, the prepared metalloporphyrin-carbon nanotube catalyst T(m-NO 2 )PPClCNTs-OH.
[0023] In the reactor, add 10mL of acetonitrile, add 2mmol cyclohexene and catalyst (0.5wt% of the substrate), charge 1.0MPa of oxygen, react at 30°C for 4 hours, the epoxide selectivity is 99%, and the raw material conversion The rate is 98%.
Embodiment 2~5
[0025] The composition of catalyst is different, and olefin epoxidation process condition is the same as embodiment 1, and the results are shown in table 1:
[0026] Table 1
[0027] Example
Embodiment 6
[0029] 1g of carbonylated carbon nanotubes was added to anhydrous DMF, and the reaction solution was ultrasonicated for 4h. After the reactants were uniformly dispersed, the metalloporphyrin (in the general formula M=Ru, R 1 =Cl,R 2 = R 3 = R 4 = R 5 =H, X=Im) 0.05g was added to the reaction solution, heated in an oil bath, and after 5h, the reaction solution was cooled to room temperature, and suction filtered with a polytetrafluoroethylene microporous membrane to obtain the prepared metalloporphyrin-carbon nanotube Catalyst T(o-Cl)RuPPImCNTs-CO.
[0030] In the reactor, add 10mL acetonitrile, add 2mmol styrene and catalyst (0.2wt% of the substrate), charge the oxygen of 0.5MPa, after reacting at 50 ℃ for 3 hours, the selectivity of epoxide is 96%, the conversion rate of raw material 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com