Method for preparing diazonium salt by utilizing micro-reactor
A technology of micro-reactor and diazonium salt, which is applied in the direction of organic chemistry, can solve the problems of blocking micro-channels, achieve continuous process, high utilization rate of raw materials, and avoid the effect of sudden temperature rise
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
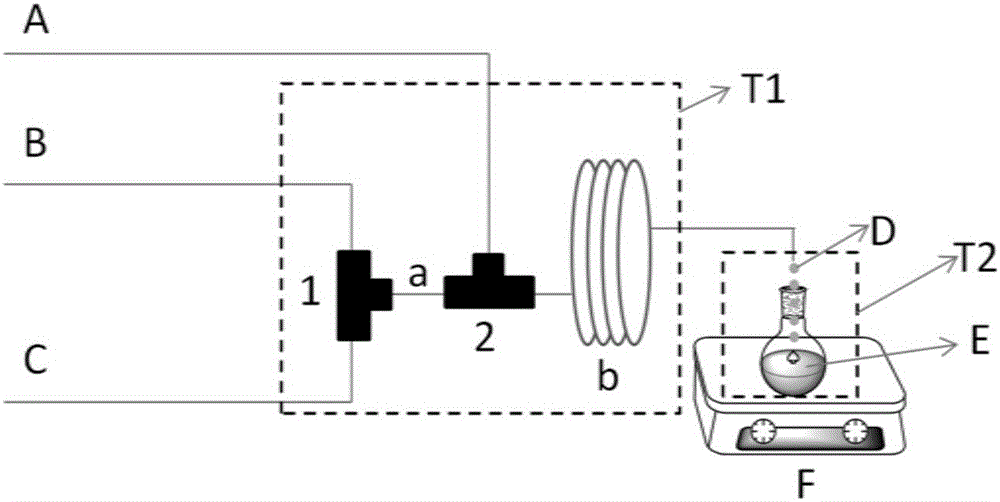
[0023] In such figure 1 In the reaction device shown, the sodium nitrite solution and o-ethylaniline are fed from B and C at flow rates of 1.1ml / min and 1ml / min respectively (the molar ratio of sodium nitrite to o-ethylaniline is 1:1) The tube is injected into the first micromixer 1 (internal interdigital micromixer, SIMM-V2, IMM, Germany, mixing channel inner diameter 45μm), and then the mixed solution passes through the first microchannel a (inner diameter: 0.3mm, residence time 0.2 s) Enter the second micromixer 2 (inner diameter 1mm) and the hydrochloric acid injected from the feed pipe A at a flow rate of 2.4ml / min (the molar ratio of hydrochloric acid to o-ethylaniline is 2.8:1) in the constant temperature reaction bath T1(- 5℃), then enter the second microchannel b (inner diameter: 1mm) to continue the reaction for 10s, the diazonium salt solution is collected in a round bottom flask E containing potassium iodide, the round bottom flask E is placed in a constant temperat...
Embodiment 2
[0025] In such figure 1 In the reaction device shown, sodium nitrite solution and o-ethylaniline are fed from B and C at flow rates of 1.44ml / min and 1ml / min (the molar ratio of sodium nitrite to o-ethylaniline is 1.05:1). The tube is injected into the first micro-mixer 1 (internal interdigital micro-mixer, SIMM-V2, IMM, Germany, mixing channel inner diameter 45μm), and then the mixed solution passes through the first microchannel a (inner diameter: 0.3mm, residence time 0.5 s) Enter the micro-second mixer 2 (inner diameter 5mm) and the hydrochloric acid injected from the feed pipe A at a flow rate of 4ml / min (the molar ratio of hydrochloric acid to o-ethylaniline is 3.2:1) in the thermostatic reaction bath T1 (0℃ ), and then enter the second microchannel b (inner diameter: 1mm) to continue the reaction for 10s, and the diazonium salt solution is collected in a round-bottom flask E containing potassium iodide. The round-bottom flask E is placed in a constant temperature reactio...
Embodiment 3
[0027] In such figure 1 In the reaction device shown, the sodium nitrite solution and o-ethylaniline are fed from B and C at flow rates of 1.44ml / min and 1ml / min (the molar ratio of sodium nitrite to o-ethylaniline is 1.1:1). The tube is injected into the first micromixer 1 (internal interdigital micromixer, SIMM-V2, IMM, Germany, the inner diameter of the mixing channel is 45μm), and then the mixed solution passes through the first microchannel a (0.3mm, residence time 0.5s) Enter the second micro-mixer 2 (inner diameter 9mm) and the hydrochloric acid injected from the feed pipe A at a flow rate of 4ml / min (the molar ratio of hydrochloric acid to o-ethylaniline is 4:1) in the thermostatic reaction bath T1 (40°C) Mix, then enter the second microchannel b (inner diameter: 1mm) to continue the reaction for 2s, and the diazonium salt solution is collected in a round-bottom flask E containing potassium iodide. The round-bottom flask E is placed in a constant temperature reaction ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com