Method for detecting residual quantity of sodium cyanoborohydride in protein sample or product
A technology of sodium cyanoborohydride and a detection method, which is applied in the direction of color/spectral characteristic measurement, etc., can solve problems such as only qualitative analysis, and achieve the effects of low cost, low toxicity, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] With reference to the above-mentioned main analysis steps, Example 1 is briefly described below.
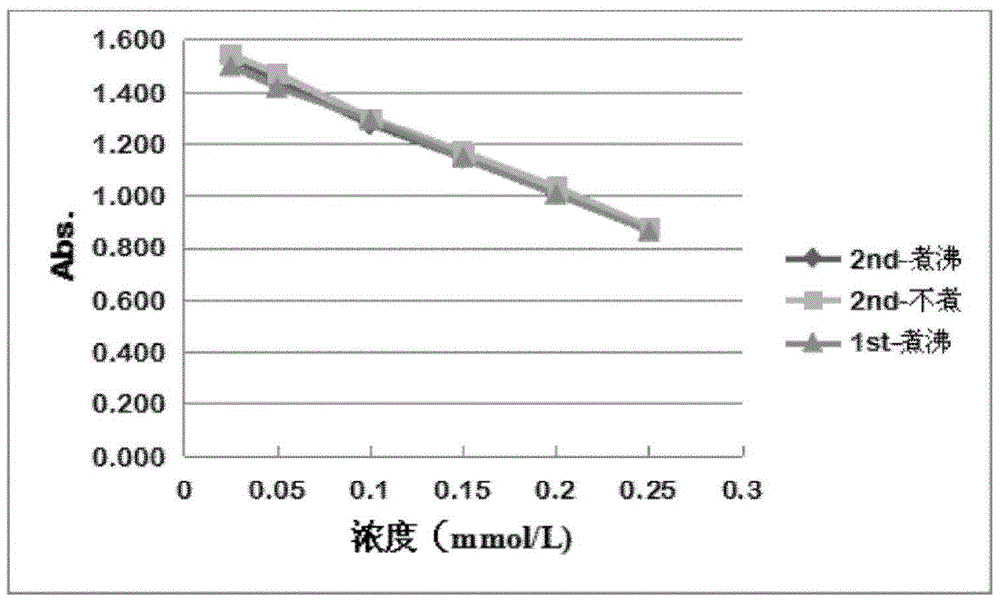
[0055] Carry out this test (check its stability) by two days:
[0056] (1) Test on the first day:
[0057] Preparation of sodium cyanoborohydride standard gradient solution: Take the standard mother liquor of sodium cyanoborohydride and dilute it with purified water to 0.25mmol / L, 0.20mmol / L, 0.15mmol / L, 0.10mmol / L, 0.05mmol / L, 0.025 mmol / L solution.
[0058] Then, put the standard solutions of different concentrations in a boiling water bath for 15 minutes, and centrifuge to get the supernatant;
[0059] Finally, the staining solution was added respectively, kept in a water bath at 37° C. for 30 min in the dark, and measured at 595 nm.
[0060] (2) Test on the second day:
[0061] Group A: Same as the first day.
[0062] Group B: The method of preparing the solution is the same as the first day, but without boiling water bath treatment; then, add the staining solutio...
Embodiment 2
[0065] With reference to the above-mentioned main analysis steps, Example 2 is briefly described below.
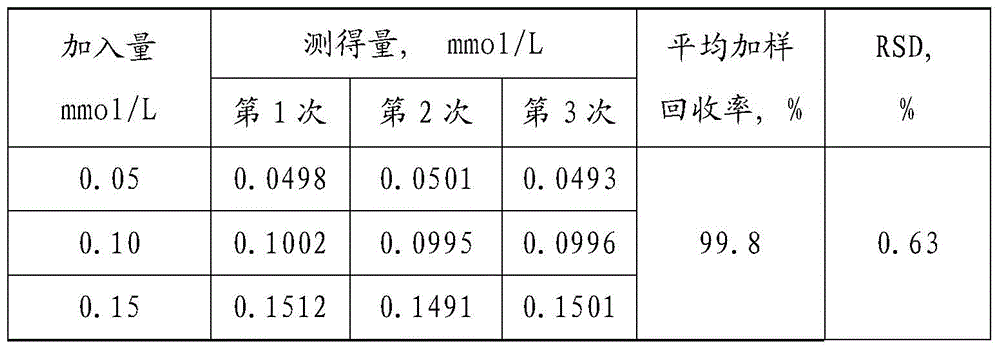
[0066] 1. Prepare standard gradient solution of sodium cyanoborohydride: take the standard mother liquor of sodium cyanoborohydride and dilute it with purified water to 0.25mmol / L, 0.20mmol / L, 0.15mmol / L, 0.10mmol / L, 0.05mmol / L ,0.025mmol / L solution.
[0067] 2. Take a batch of PEG-rhG-CSF stock solution (pure stock solution, without sodium cyanoborohydride), perform pretreatment (to remove PEG-rhG-CSF protein), and take the supernatant after centrifugation;
[0068] Take three supernatants and add 1mmol / L standard sodium cyanoborohydride solution respectively to make the final concentrations respectively 0.05mmol / L, 0.10mmol / L, and 0.15mmol / L.
[0069] 3. Add the staining solution respectively, keep in a water bath at 37°C for 30 minutes in the dark, and measure at 595nm. The measurement results are shown in Table 1 below.
[0070] Table 1 embodiment 1 sample addition ...
Embodiment 3
[0073] With reference to the above-mentioned main analysis steps, Example 2 is briefly described below.
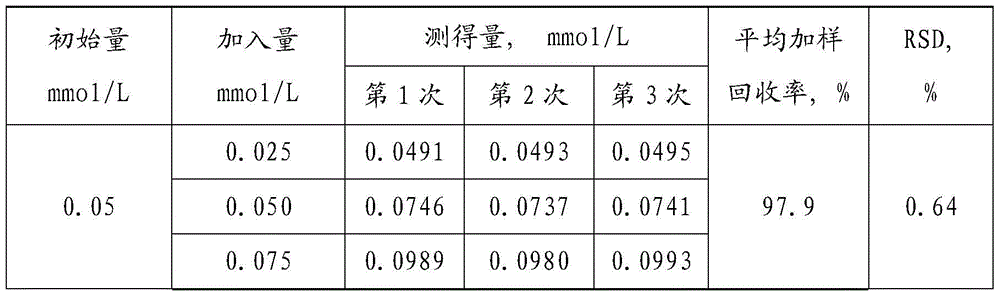
[0074] 1. Prepare standard gradient solution of sodium cyanoborohydride: take the standard mother liquor of sodium cyanoborohydride and dilute it with purified water to 0.25mmol / L, 0.20mmol / L, 0.15mmol / L, 0.10mmol / L, 0.05mmol / L ,0.025mmol / L solution.
[0075] 2. Take the rhG-CSF stock solution (pure stock solution, without sodium cyanoborohydride), add 20mmol / L sodium cyanoborohydride to obtain rhG-CSF sample;
[0076] Sample pretreatment (removal of rhG-CSF protein), supernatant after centrifugation;
[0077] Then the obtained sample is diluted 400 times (purified water dilution), that is, the sample contains 0.05mmol / L sodium cyanoborohydride;
[0078] Divide the obtained sample into three parts A, B, and C according to the volume ratio of 1:1:1. Add an equal volume of 0.05mmol / L standard sodium cyanoborohydride solution to A sample, and add 0.10mmol / L standard cyanobo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com