Core-shell Cu@Au catalyst as well as preparation method and application thereof
A catalyst and core-shell technology, which is used in the preparation of lactic acid, catalytic oxidation of 1,2-propanediol, bimetallic application of precious metals and non-precious metals, can solve the problems of high cost and difficulty in meeting industrial application requirements, and achieve reduction Catalyst cost, good industrial application value, high catalytic activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Catalyst preparation:
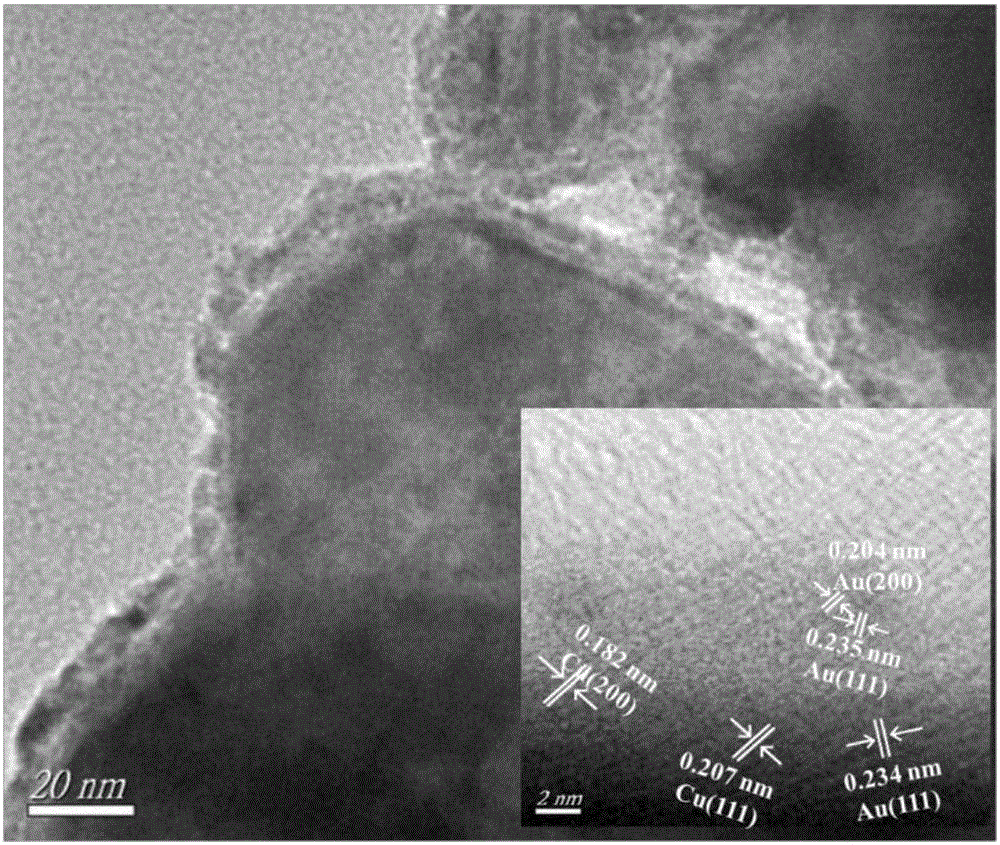
[0032] The core-shell CuAu nanocatalysts were prepared by reducing copper nitrate and chloroauric acid with hydrazine hydrate. Dissolve 3.78g of copper nitrate trihydrate and 0.378g of Tween in 120ml of absolute ethanol, and ultrasonically dissolve them for 30 minutes to form a mixed solution. When the temperature of the mixed solution rises to 60°C, add 1.5mol / L NaOH ethanol solution drop by drop to adjust the mixed solution The pH value is between 8~9; Then dropwise add dilute ethanol solution of hydrazine hydrate (hydrazine hydrate / 160ml dehydrated alcohol of 16ml85%), and react 2h under magnetic stirring, the nano-copper prepared will be cooled to 30°C; Weigh 0.1g of chloroauric acid tetrahydrate and dissolve it in 20ml of absolute ethanol, add the ethanol solution of chloroauric acid dropwise into the cooled mixture to react for 1 hour. Finally, centrifuge, wash with absolute ethanol, and dry to obtain the core-shell Cu 0.985 Au 0.015 Nan...
Embodiment 2
[0036] Adopt the same catalyst preparation method of embodiment 1, only change the consumption of chloroauric acid tetrahydrate to be 0g, 0.03g, 0.23g and 0.34g, prepare nanocatalyst Cu, Cu 0.995 Au 0.005 、Cu 0.965 Au 0.035 and Cu 0.95 Au 0.05 . Au nanoparticles were prepared by reducing 0.34g chloroauric acid tetrahydrate with hydrazine hydrate in the presence of Tween. The Cu 0.995 Au 0.005 、Cu 0.985 Au 0.015 、Cu 0.965 Au 0.035 and Cu 0.95 Au 0.05 The ratio in the subscript is the molar ratio of Cu and Au.
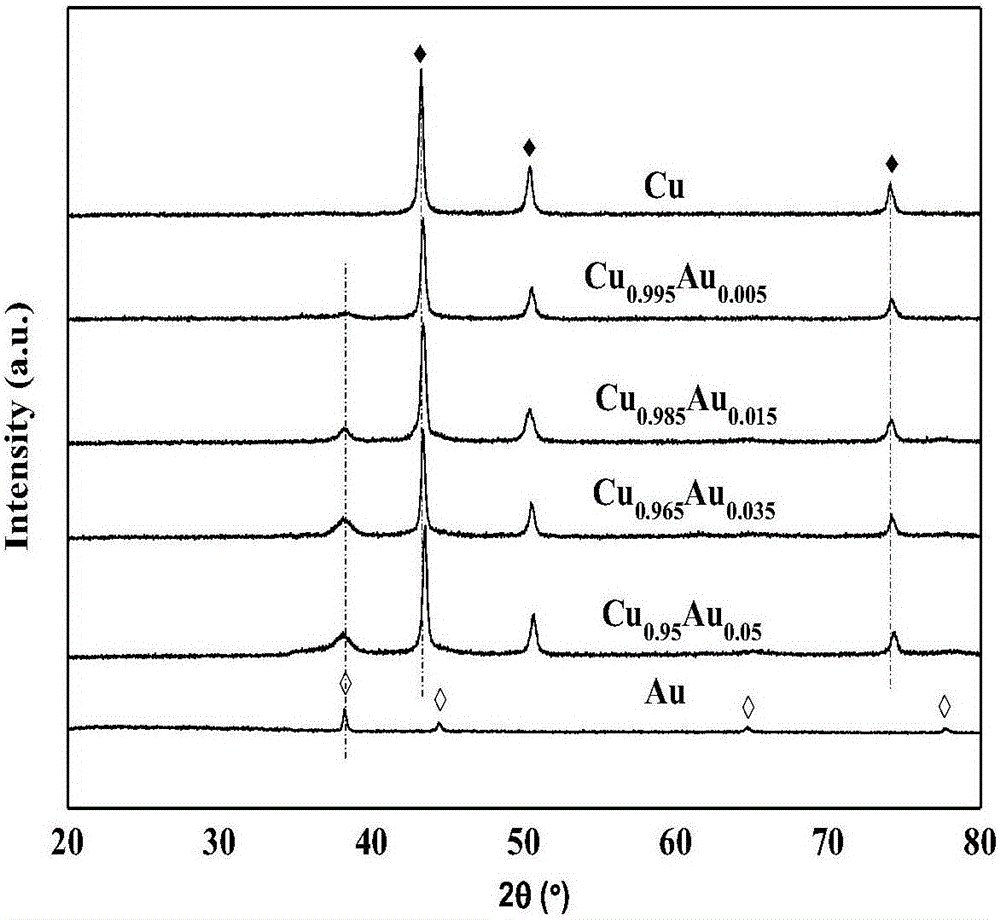
[0037] figure 1XRD spectra of pure-phase Cu, Au catalysts and CuAu nanobimetallic catalysts with different molar ratios. It can be seen from the XRD spectrum that the pure-phase Cu sample is basically consistent with the data (2θ=43.3, 50.4, 74.1°) on the JCPDS card 04-0836, and the sample is a face-centered cubic pure-phase elemental copper; the same pure-phase Au The sample basically agrees with the data on JCPDS card 46-1043 (2θ=38.2, 44.4, 64.6, 77.5°...
Embodiment 3
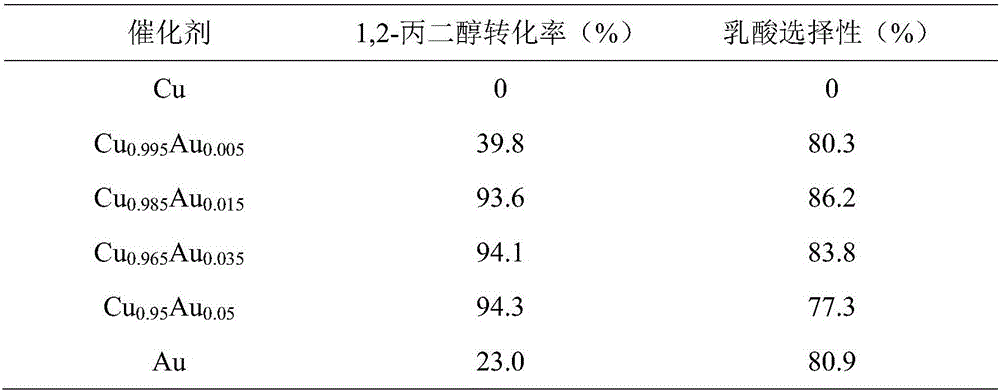
[0043] Adopt the same method of embodiment 1 to prepare core-shell Cu 0.985 Au 0.015 Nano-catalyst, the process of catalytic oxidation of 1,2-propanediol adopts the same method as in Example 1, only changing the reaction time to 0.5h, 1h, 2h, and 3h respectively, can obtain different reaction times for catalytic oxidation of 1,2-propanediol The impact of the reaction, the results are shown in Table 2, as the reaction time prolongs, the conversion rate of 1,2-propanediol obviously increases, while the selectivity of lactic acid decreases.
[0044] Table 2 Effect of reaction time on 1,2-propanediol catalytic oxidation reaction
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com