Preparation method and medical application of berberine hydrochloride conjugate
A technology of berberine hydrochloride and acid compound, applied in the field of food and pharmacy, can solve the problems of low oral bioavailability, low fat solubility, poor absorption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
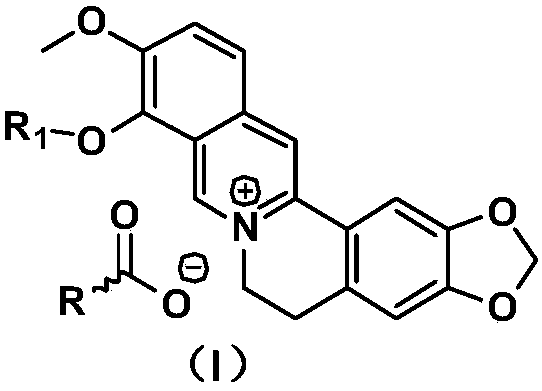
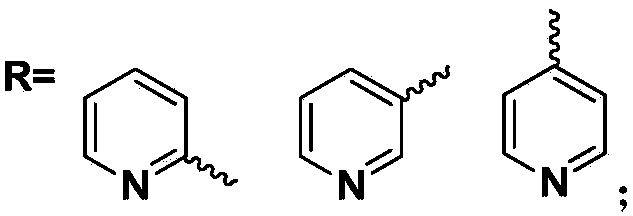
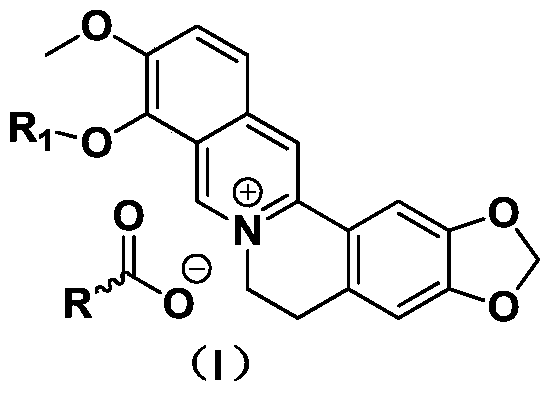
[0020] Synthesis of nicotinic acid berberine conjugate
[0021] Take 3.7g of berberine hydrochloride, put it into a 100ml three-necked flask, add 100ml of ethanol, adjust the pH to 7-8 with 0.5mol / l sodium hydroxide, raise the temperature to 60-70°C, stir to dissolve, then add 1.25g of nicotinic acid , keep stirring at this temperature for 1-2 hours, filter while hot, concentrate the filtrate to one-third of the original volume, cool and crystallize, filter and dry to obtain 3.9 g of nicotinic acid berberine conjugate with a yield of 85%.
Embodiment 2
[0023] Synthesis of nicotinic acid berberine conjugate
[0024] Take 3.7g of berberine hydrochloride, put it into a 100ml three-neck flask, add 150ml of ethanol, adjust the pH to 7-8 with 0.6mol / l sodium hydroxide, raise the temperature to 60-70°C, stir to dissolve, then add 1.3g of nicotinic acid , keep stirring at this temperature for 1-2 hours, filter while hot, concentrate the filtrate to one-third of the original volume, cool and crystallize, filter and dry to obtain 4.1 g of nicotinic acid berberine conjugate with a yield of 89.5%.
Embodiment 3
[0026] Synthesis of nicotinic acid berberine conjugate
[0027] Take 3.7g of berberine hydrochloride, put it into a 100ml three-necked flask, add 100ml of ethanol, adjust the pH to 7-8 with 3mol / l sodium hydroxide, raise the temperature to 60-70°C, stir to dissolve, then add 2.0g of nicotinic acid, Stir at this temperature for 1-2 hours, filter while hot, concentrate the filtrate to one-third of the original volume, cool and crystallize, filter, and dry to obtain 4.0 g of nicotinic acid berberine conjugate with a yield of 87.3%. ESI-MS (M + +H)m / zcalcdforC 20 h 16 NO 4 + 337.13found337.17; ESI-MS (M + +H)m / zcalcdforC 6 h 4 NO 2 - 122.02found122.05.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com