Method for preparing arylamine by reducing aromatic nitro compound
An aromatic nitro compound technology is applied in the field of aromatic nitro compound reduction to prepare aromatic amine, and achieves the effects of good reaction selectivity, less waste liquid discharge and simple treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
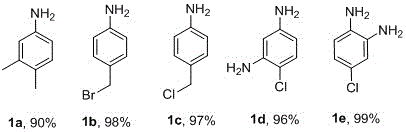
[0021] Take 1mmol of 3,4-dimethylnitrobenzene (151.2mg), 4-nitrobenzyl bromide (216.0mg), 4-nitrochlorobenzene (157.6mg), 2-chloro-5-nitroaniline (172.6 mg), 4-chloro-2-nitroaniline; one of (172.6 mg), 0.3 mmol NaOH (12.0 mg), 1% mmol Pd(OAc) 2 (2.3mg), ionic liquid (100mg), H 2 Add 1.5mL of O1.5mL to the pressure tube and stir evenly, add 5mmol hydrazine hydrate, react in 50°C oil bath for 20h, add 2mL ethyl acetate to extract three times after the reaction is stopped, remove the organic solvent by rotary evaporation, and pass through column chromatography on silica gel , to obtain pure target products 1a-1e, the yields are 90%, 98%, 97%, 96%, 99%, respectively, as shown below.
[0022]
Embodiment 2
[0024] Take 1mmol of p-nitroanisole (153.1mg), m-nitroaniline (138.1mg), o-fluoronitrobenzene (141.1mg), 4-fluoro-3-nitrotoluene (155.1mg) respectively , 0.3mmolKOH(16.9mg), 1%mmolPd(OAc) 2 (2.3mg), ionic liquid (100mg), H 2 Add 1.5mL of O1.5mL to the pressure tube and stir evenly, add 5mmol hydrazine hydrate, react in 50°C oil bath for 20h, add 2mL ethyl acetate to extract three times after the reaction is stopped, remove the organic solvent by rotary evaporation, and pass through column chromatography on silica gel , the pure target products 2a-2d were obtained, and the yields were 95%, 98%, 98%, and 85%, respectively, as shown below.
[0025]
Embodiment 3
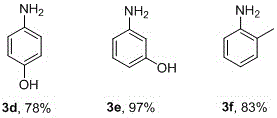
[0027] Take 1mmol of 6-nitroquinoline (174.2mg), p-bromonitrobenzene (202.01mg), p-chloronitrobenzene (157.6mg), p-nitrophenol (139.1mg), m-nitrophenol (139.1mg) , one of o-nitrotoluene (137.1mg), 0.3mmolK 2 CO 3 (41.5mg), 1%mmolPd(OAc) 2 (2.3mg), ionic liquid (100mg), H 2 Add 1.5mL of O1.5mL to the pressure tube and stir evenly, add 5mmol hydrazine hydrate, react in 50°C oil bath for 20h, add 2mL ethyl acetate to extract three times after the reaction is stopped, remove the organic solvent by rotary evaporation, and pass through column chromatography on silica gel , the pure target products 3a-3f were obtained, and the yields were 94%, 90%, 99%, 78%, 97%, 83%, respectively, as shown below.
[0028]
[0029]
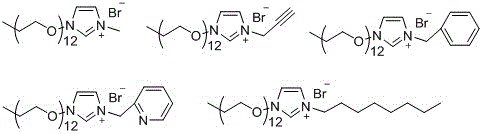
[0030] The inventors also synthesized the following ionic liquids:
[0031]
[0032] And they were applied to the reduction reaction of aromatic nitro compounds. It was found that the ionic liquid formed by connecting different groups of imidazole 3 had diff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com