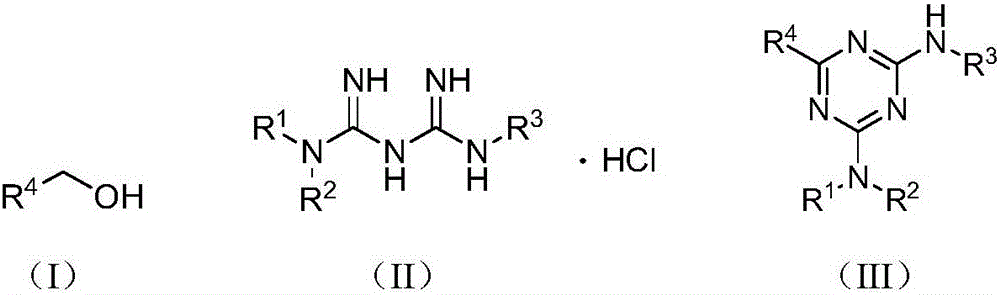
Preparation method of s-triazine compound
A compound, the technology of s-triazine, which is applied in the field of preparation of s-triazine compounds, achieves the effects of low cost, wide industrial application prospect and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
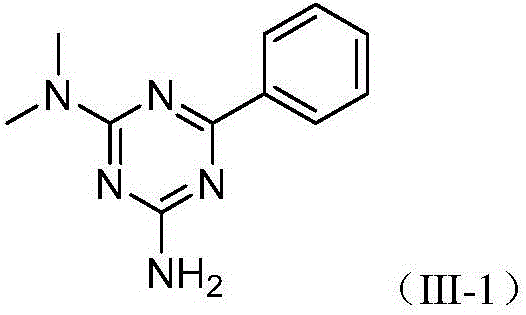
[0018] The present invention will be further described below through specific examples, but the protection scope of the present invention is not limited thereto. Embodiment 1: the preparation of compound (III-1)
[0019] In a reaction vessel, add metformin hydrochloride (0.1653g, 0.9954mmol), benzyl alcohol (0.1082g, 1.0002mmol), 1,5-cyclooctadiene ruthenium dichloride (0.0056g, 0.02mmol), tert-butyl Potassium alcoholate (0.2250g, 2.0052mmol), 1,4-dioxane (4mL), stirred and reacted in an oil bath at 100°C for 15 hours; after the reaction, add water (10mL), extract with ethyl acetate (10mL× 3), the organic layers were combined, dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated, and the obtained concentrate was separated by column chromatography, and the eluent was dichloromethane:methanol=50:1 (V:V), and R f The eluate with a value of 0.3-0.35 (monitored by TLC, the developing solvent is the same as the eluent) was distilled under reduced pressur...
Embodiment 2
[0023] Change 1,5-cyclooctadiene ruthenium dichloride to dodecacarbonyl ruthenium (0.0128g, 0.02mmol), other operations are the same as in Example 1, and finally obtain the target compound (III-1) 0.1586g, the yield is 74 %.
Embodiment 3
[0025] 1,5-Cyclooctadiene ruthenium dichloride was changed to triphenylphosphine ruthenium chloride (0.0182g, 0.02mmol), and other operations were the same as in Example 1 to finally obtain 0.1180g of the target compound (III-1). The rate is 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com