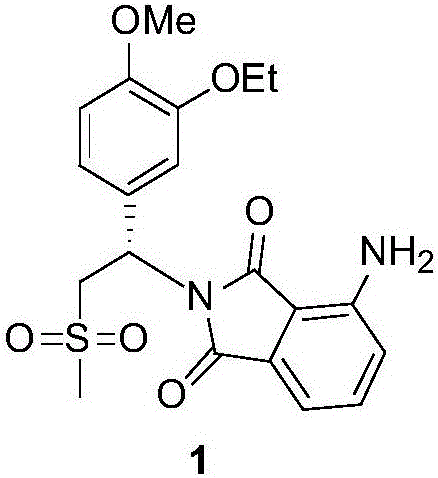
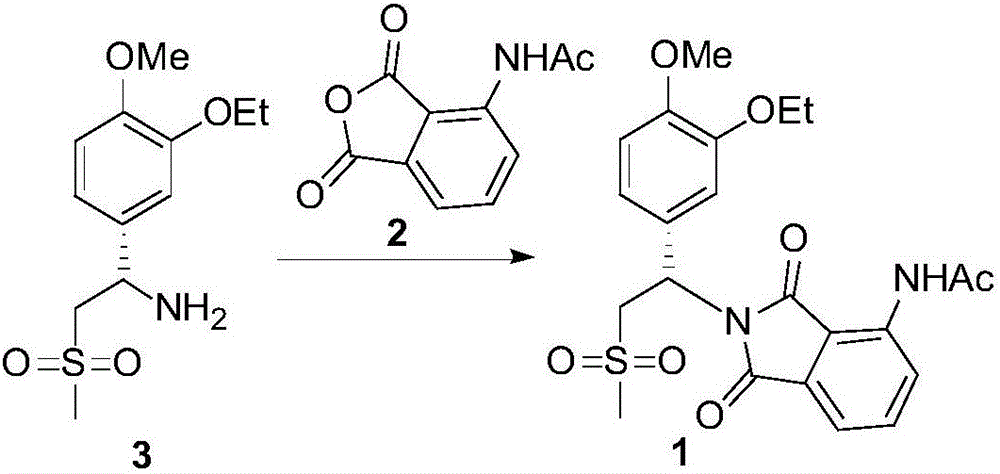
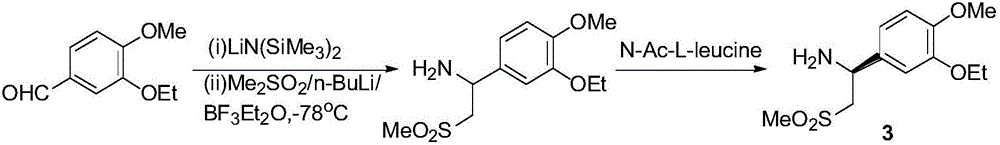
Synthesis process of apremilast intermediate
A synthesis process and a technology for intermediates, which are applied in the field of synthesis technology of Aprestel intermediates, can solve the problems of complicated post-processing, difficult operation, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0042] Step a: Preparation of 4-[(1-chloro-2-methylsulfonyl)-ethyl]-2-ethoxy-1-methoxybenzene (5)
[0043] In a 500mL reaction flask, 17.8g (100mmol) of 3-ethoxy-4-methoxystyrene (6) was dissolved in 267g of toluene, and 0.96g of tris(triphenylphosphine)ruthenium chloride (1mmol) was added and 17.2g (150mmol) of methanesulfonyl chloride was heated to 110°C under the protection of nitrogen, cooled to room temperature after 20h of reaction, washed with water and brine, and evaporated to dryness to obtain an oily substance with a HPLC purity of 82%, which was directly put into the next step without purification.
[0044] Step b: Preparation of 1-(3-ethoxy-4-methoxy)phenyl-2-methanesulfonylethylamine (4)
[0045] Add 176g of ammonia-methanol saturated solution into a 500mL reaction flask, add 1.0g of triethylamine and 0.15g of sodium iodide, heat to 50°C under airtight conditions, and dissolve the oily 4-[(1-chloro-2- Methanesulfonyl)-ethyl]-2-ethoxy-1-methoxybenzene (5) was diss...
Embodiment example 2
[0049] Step a: Preparation of 4-[(1-chloro-2-methylsulfonyl)-ethyl]-2-ethoxy-1-methoxybenzene (5)
[0050] In a 500mL reaction flask, 17.8g (100mmol) of 3-ethoxy-4-methoxystyrene (6) was dissolved in 267g of toluene, and 0.96g of tris(triphenylphosphine)ruthenium chloride (1mmol) was added and 17.2g (150mmol) of methanesulfonyl chloride was heated to 90°C under the protection of nitrogen, cooled to room temperature after 12 hours of reaction, washed with water and brine, and evaporated to dryness to obtain an oily substance with a HPLC purity of 79%, which was directly put into the next step without purification.
[0051] Step b: Preparation of 1-(3-ethoxy-4-methoxy)phenyl-2-methanesulfonylethylamine (4)
[0052] Add 176g of ammonia-methanol saturated solution into a 500mL reaction flask, add 1.0g of triethylamine and 0.15g of sodium iodide, heat to 40°C under airtight conditions, and dissolve the oily 4-[(1-chloro-2- Methanesulfonyl)-ethyl]-2-ethoxy-1-methoxybenzene (5) was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com