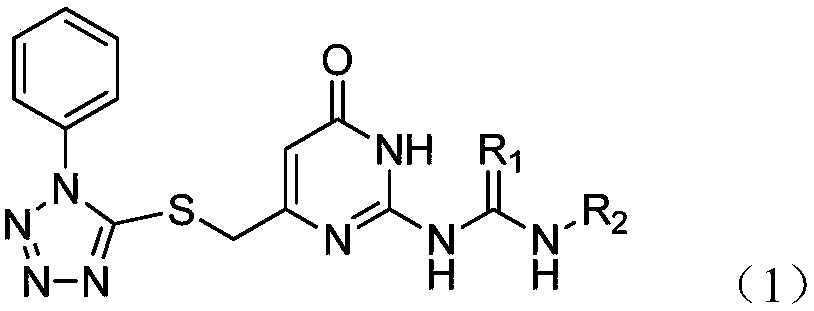
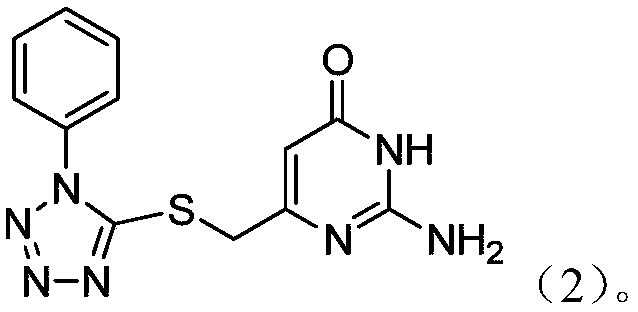
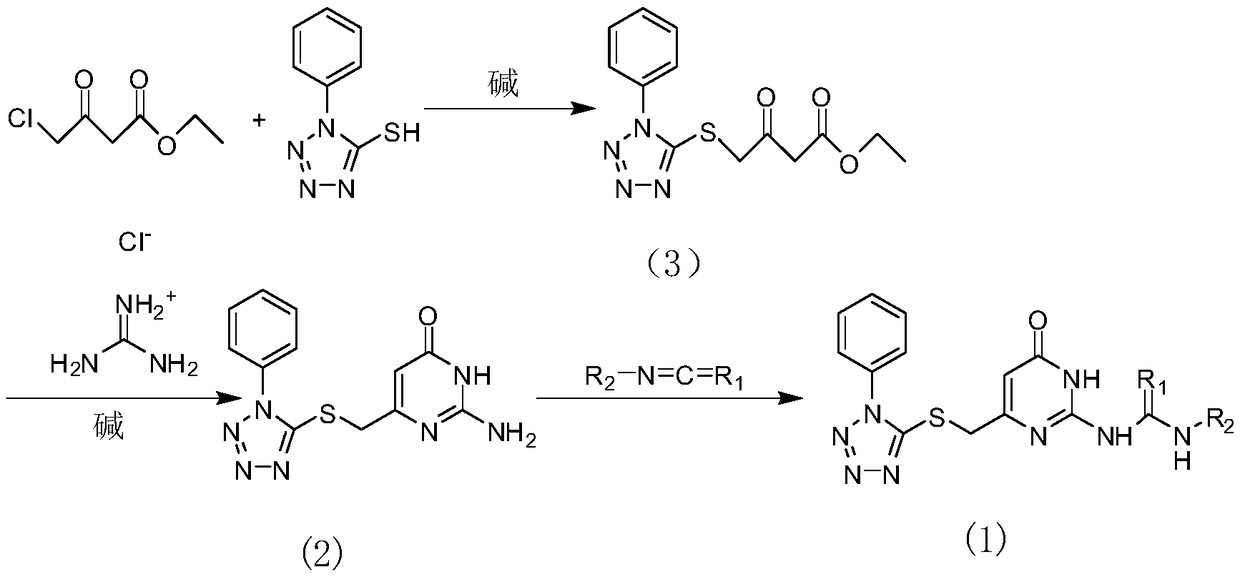
Potential ezh2 small molecule inhibitors and methods for their synthesis
A technology of a small molecule inhibitor and a synthesis method, which is applied in the field of medicine and chemical industry, and achieves the effects of simple synthesis route, simple separation process and good EZH2 inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] FTCI-2362
[0032]
[0033] Take 1.78g (0.01mol) of 1-phenyl-5-mercapto-1H-tetrazolium into a round bottom flask, add 30ml of methanol dropwise, and stir until the white solid is completely dissolved. Under stirring, 1.81 g (0.011 mol) of ethyl 4-chloroacetoacetate was added dropwise and stirred at room temperature for 5 h, and the spot reaction was complete. Spin the methanol to dryness under reduced pressure, add 5ml of deionized water to the residual oil to dissolve, then add ethyl acetate to extract the water phase three times, combine the organic phases, dry, and recover the ethyl acetate under reduced pressure to obtain 2.82g of a colorless oily liquid (93%).
[0034] Take 1.5g (4.9×10-3mol) of the oil obtained in the previous step, add an appropriate amount of absolute ethanol and stir, add 0.94g (9.8×10-3mol) of guanidine hydrochloride under stirring, add 0.53g (9.8×10-3mol) After the ethanol solution of sodium methoxide was heated to reflux for 8 hours, th...
Embodiment 2
[0037] FTCI-2377
[0038]
[0039] Take 1.78g (0.01mol) of 1-phenyl-5-mercapto-1H-tetrazolium into a round bottom flask, add 30ml of methanol dropwise, and stir until the white solid is completely dissolved. Under stirring, 1.81 g (0.011 mol) of ethyl 4-chloroacetoacetate was added dropwise and stirred at room temperature for 5 h, and the spot reaction was complete. Spin the methanol to dryness under reduced pressure, add 5ml of deionized water to the residual oil to dissolve, then add ethyl acetate to extract the water phase three times, combine the organic phases, dry, and recover the ethyl acetate under reduced pressure to obtain 2.82g of a colorless oily liquid (93%).
[0040] Take 1.5g (4.9×10-3mol) of the oil obtained in the previous step, add an appropriate amount of absolute ethanol and stir, add 0.94g (9.8×10-3mol) of guanidine hydrochloride under stirring, add 0.53g (9.8×10-3mol) After the ethanol solution of sodium methoxide was heated to reflux for 8 hours, th...
Embodiment 3
[0043] FTCI-2369
[0044]
[0045] Take 1.78g (0.01mol) of 1-phenyl-5-mercapto-1H-tetrazolium into a round bottom flask, add 30ml of methanol dropwise, and stir until the white solid is completely dissolved. Under stirring, 1.81 g (0.011 mol) of ethyl 4-chloroacetoacetate was added dropwise and stirred at room temperature for 5 h, and the spot reaction was complete. Spin the methanol to dryness under reduced pressure, add 5ml of deionized water to the residual oil to dissolve, then add ethyl acetate to extract the water phase three times, combine the organic phases, dry, and recover the ethyl acetate under reduced pressure to obtain 2.82g of a colorless oily liquid (93%).
[0046] Take 1.5g (4.9×10-3mol) of the oil obtained in the previous step, add an appropriate amount of absolute ethanol and stir, add 0.94g (9.8×10-3mol) of guanidine hydrochloride under stirring, add 0.53g (9.8×10-3mol) After the ethanol solution of sodium methoxide was heated to reflux for 8 hours, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com