Amphipathic phospholipid molecule with reducing response and application thereof in drug sustained release
An amphiphilic phospholipid, responsive technology, which can be used in medical preparations with non-active ingredients, phosphorus-organic compounds, organic chemistry, etc., and can solve the problems of limited use of reducing agents, difficulty in separation and purification, and cumbersome synthesis of reduction-responsive vesicles.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0034]
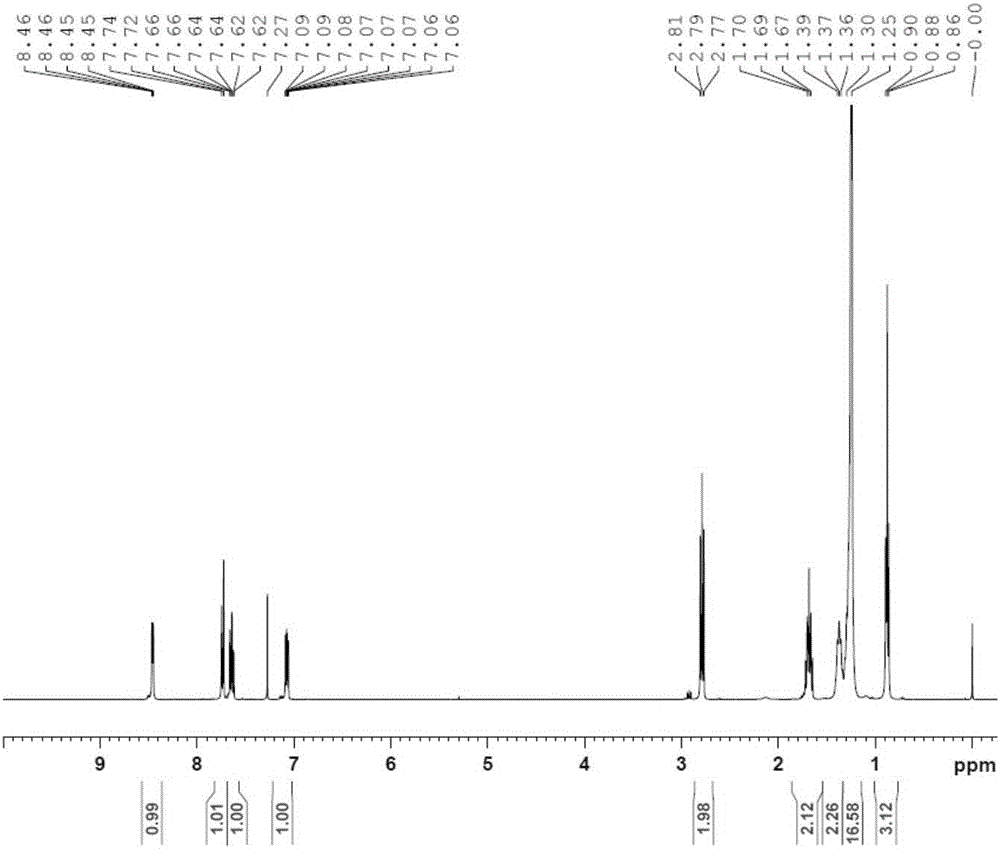
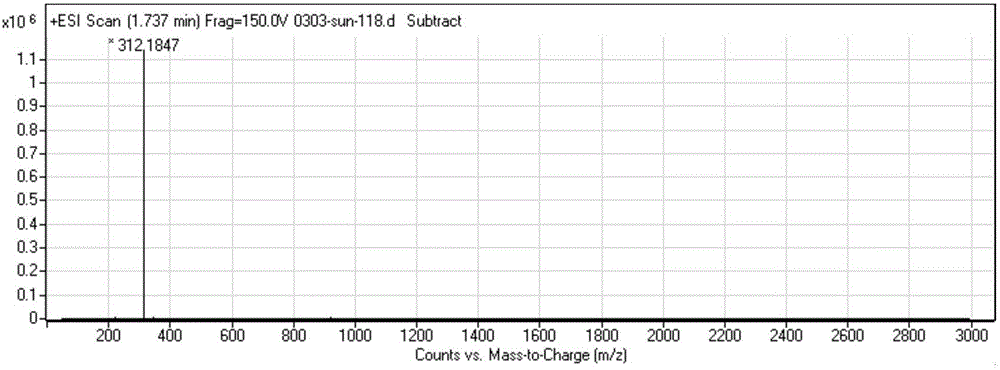
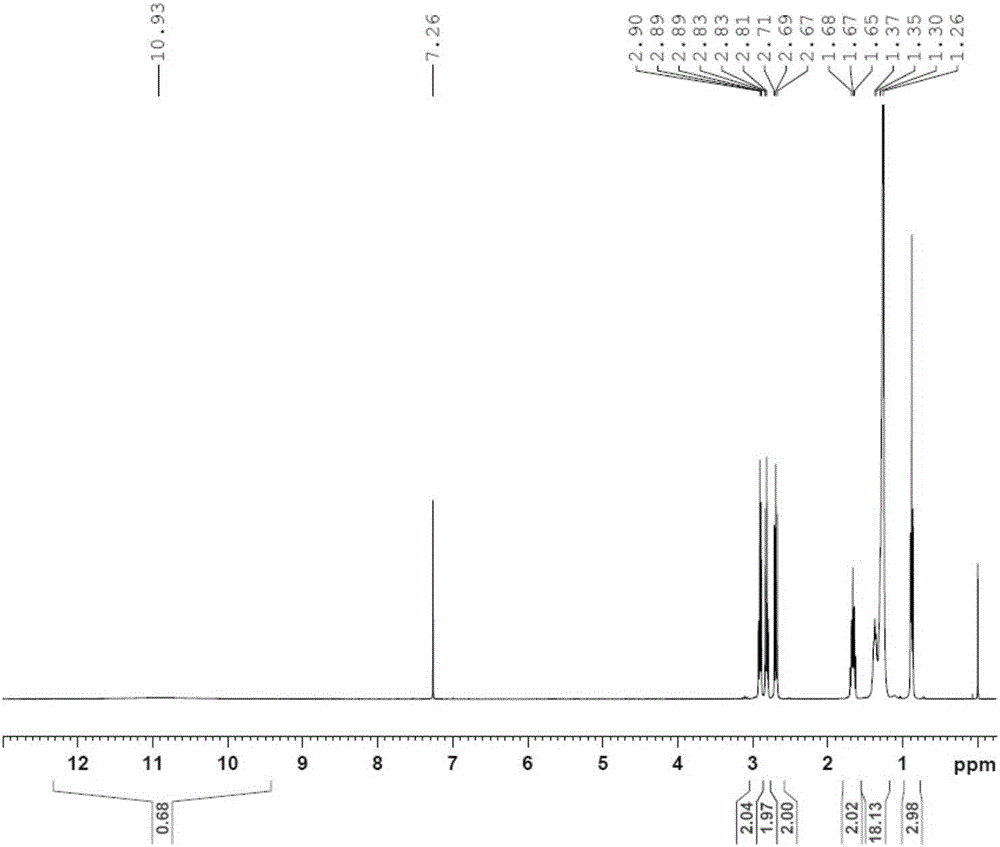
[0035] Synthetic route: a. 2,2'-dithiodipyridine, triethylamine, 0°C; b. Mercaptopropionic acid, triethylamine, dichloromethane, 25°C; c. 1-hexadecanoyl-glycerol - Phosphocholine, dicyclohexylcarbodiimide, dichloromethane, 25°C.
[0036] Step a: Dissolve 20 g of dodecylmercaptan and 10 g of triethylamine in 200 ml of dichloromethane, add a solution of 88 g of 2,2'-dithiobipyridine in 100 ml of dichloromethane with stirring in an ice bath , the reaction solution was stirred at 0° C. for 1 hour, then naturally rose to room temperature, and continued to stir for 24 hours. After the reaction was finished, the reaction solution was concentrated until a large amount of crystals appeared, and suction filtered. The filter cake was washed with 200 ml of n-hexane, and the combined filtrate was crystallized with 50 ml of glacial ether to obtain 25 g of compound 2 (light yellow solid), with a yield of 79%;
[0037] Step b: 15.5 grams of compound 2 and 5.3 grams of mercaptopro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com